UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C., 20549
FORM 10-K
(Mark One)
| x | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended December 31, 2010
OR
| ¨ | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
Commission File Number: 000-51902
INFUSYSTEM HOLDINGS, INC.
(Exact Name of Registrant as Specified in its Charter)
| Delaware | 20-3341405 | |
| (State or Other Jurisdiction of Incorporation or Organization) |
(I.R.S. Employer Identification No.) |
31700 Research Park Drive
Madison Heights, Michigan 48071
(Address of Principal Executive Offices) (Zip Code)
Registrant’s Telephone Number, including Area Code:
(248) 291-1210
Securities Registered Pursuant to Section 12(b) of the Act:
| Title of Each Class |
Name of Exchange on which Registered | |
| Common Stock, par value $0.0001 per share | New York Stock Exchange Amex |
Securities Registered Pursuant to Section 12(g) of the Act:
None
(Title of Class)
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. YES ¨ NO x
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. YES ¨ NO x
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter periods as the registrant was required to file such reports) and (2) has been subject to such filing requirements for the past 90 days. YES x NO ¨
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Website, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). YES ¨ NO ¨
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K is not contained herein, and will not be contained, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. ¨
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act. (check one)
| Large accelerated filer | ¨ | Accelerated filer ¨ | ||
| Non-accelerated filer | ¨ | Smaller reporting company x | ||
| (Do not check if smaller reporting company.) | ||||
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Act). YES ¨ NO x
The aggregate market value of the registrant’s voting equity held by non-affiliates of the registrant, computed by reference to the closing sales price for the registrant’s common stock on June 30, 2010, as reported on the OTC Bulletin Board, was approximately $40,828,912. In determining the market value of the voting equity held by non-affiliates, securities of the registrant beneficially owned by directors and officers of the registrant have been excluded. This determination of affiliate status is not necessarily a conclusive determination for other purposes.
The number of shares of the registrant’s common stock outstanding as of March 9, 2011 was 21,105,506.
DOCUMENTS INCORPORATED BY REFERENCE
Portions of this registrant’s definitive proxy statement for its 2011 Annual Meeting of Stockholders to be filed with the SEC no later than 120 days after the end of the registrant’s fiscal year are incorporated herein by reference in Part III of this Annual Report on Form 10-K.
Cautionary Statement about Forward-Looking Statements
This Annual Report on Form 10-K includes forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended (the “Securities Act”) and Section 21E of the Securities Exchange Act of 1934, as amended (the “Exchange Act”). All statements other than statements of historical facts contained in this Annual Report on Form 10-K, including statements regarding the future financial position, business strategy, plans, and objectives of management for future operations, are forward-looking statements. The words “believe,” “may,” “will,” “estimate,” “continue,” “anticipate,” “intend,” “should,” “plan,” “expect,” and similar expressions, as they relate to us, are intended to identify forward-looking statements. We have based these forward-looking statements largely on current expectations and projections about future events and financial trends that we believe may affect financial condition, results of operations, business strategy and financial needs. These forward-looking statements are subject to a number of risks, uncertainties and assumptions, including, without limitation, those described in “Risk Factors” and elsewhere in this Annual Report on Form 10-K, including, among other things:
| • | dependence on our Medicare Supplier Number; |
| • | changes in third-party reimbursement rates; |
| • | availability of chemotherapy drugs used in our infusion pump systems; |
| • | physician’s acceptance of infusion pump therapy over oral medications; |
| • | our growth strategy, involving entry into new fields of infusion-based therapy; |
| • | the current global financial crisis; |
| • | industry competition; and |
| • | dependence upon our suppliers. |
These risks are not exhaustive. Other sections of this Annual Report on Form 10-K include additional factors which could adversely impact our business and financial performance. Moreover, we operate in a very competitive and rapidly changing environment. New risk factors emerge from time to time and it is not possible for us to predict all risk factors, nor can we assess the impact of all factors on our business or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in any forward-looking statements.
You should not rely upon forward looking statements as predictions of future events. We cannot assure you that the events and circumstances reflected in the forward looking statements will be achieved or occur. Although we believe that the expectations reflected in the forward looking statements are reasonable, we cannot guarantee future results, levels of activity, performance or achievements.
1
References in this Annual Report on Form 10-K to “we,” “us,” or the “Company” are to InfuSystem Holdings, Inc. and its subsidiaries.
| Item 1. | Business. |
Background
We were formed as a Delaware blank check company in 2005 for the purpose of acquiring through a merger, capital stock exchange, asset acquisition or other similar business combination, one or more operating businesses in the healthcare sector. We completed our initial public offering on April 18, 2006. On September 29, 2006, we entered into a Stock Purchase Agreement (as, amended the “Stock Purchase Agreement”) with I-Flow Corporation (I-Flow), Iceland Acquisition subsidiaries, our wholly-owned subsidiaries, and InfuSystem, a wholly-owned subsidiary of I-Flow Corporation. Upon the closing of the transactions contemplated by the Stock Purchase Agreement on October 25, 2007, Iceland Acquisition subsidiaries purchased all of the issued and outstanding capital stock of InfuSystem from I-Flow and concurrently merged with and into InfuSystem. As a result of the merger, Iceland Acquisition subsidiaries ceased to exist as an independent entity and InfuSystem, as the corporation surviving the merger, became our wholly-owned subsidiary Effective October 25, 2007, we changed our corporate name from HAPC, INC. to InfuSystem Holdings, Inc.
InfuSystem was incorporated under the laws of the State of California in December 1997 under the name I-Flow subsidiary, Inc., as a wholly owned subsidiary of I-Flow. In February 1998, I-Flow subsidiary, Inc. acquired Venture Medical, Inc. and InfuSystem II, Inc. in a merger transaction pursuant to which I-Flow subsidiary, Inc. as the surviving corporation changed its name to InfuSystem, Inc.
Business Concept and Strategy
The Company is the leading provider of infusion pumps and related services. The Company services hospitals, oncology practices and other alternate site healthcare providers. Headquartered in Madison Heights, Michigan, the Company delivers local, field-based customer support, and also operates pump service and repair Centers of Excellence in Michigan, Kansas, California, and Ontario, Canada.
Our core service is to supply electronic ambulatory infusion pumps and associated disposable supply kits to oncology practices, infusion clinics and hospital outpatient chemotherapy clinics to be utilized in the treatment of a variety of cancers including colorectal cancer. Colorectal cancer (CRC) is the second most prevalent form of cancer in the United States, according to the American Cancer Society, and the standard of care for the treatment of CRC relies upon continuous chemotherapy infusions delivered via electronic ambulatory infusion pumps.
The Company provides these pumps and related supplies to oncology clinics, obtains an assignment of insurance benefits from the patient, and bills the patient’s insurance company or patient as appropriate, for the use of the pump and supplies, and collects payment. The Company provides pump management services for the pumps and associated disposable supply kits to over 1,300 oncology practices in the United States. The Company retains title to the pumps during this process.
In addition, the Company sells, rents and leases new and pre-owned pole mounted and ambulatory infusion pumps to, and provides biomedical certification, maintenance and repair services for, these same oncology practices as well as to other alternate site settings including home care and home infusion providers, skilled nursing facilities, pain centers and others in the United States and Canada. The Company also provides these products and services to customers in the hospital market.
The Company purchases new and pre-owned pole mounted and ambulatory infusion pumps from a variety of sources on a non-exclusive basis. The Company repairs, refurbishes and provides biomedical certification for the devices as needed. The pumps are then available for sales, rental or to be used within the Company’s ambulatory infusion pump management service.
2
One aspect of our business strategy over the next one to three years is to expand into treatment of other cancers. We currently generate approximately 20% of our revenue from treatments for disease states other than colorectal cancer. There are a number of approved treatment regimens for head and neck, pancreatic, esophageal and other gastric cancers which present opportunities for growth. There are also a number of other drugs currently approved by the U.S. Food and Drug Administration (the FDA), as well as agents in the pharmaceutical development pipeline, which we believe could potentially be used with continuous infusion protocols for the treatment of other diseases in addition to colorectal cancer. Drugs or protocols currently in clinical trials may also obtain regulatory approval over the next several years. If these new drugs obtain regulatory approval for use with continuous infusion protocols, we expect the pharmaceutical companies to focus their sales and marketing forces on promoting the new drugs and protocols to physicians.
Another aspect of our business strategy over the next one to three years is to actively pursue opportunities for the expansion of our business through strategic alliances, joint ventures and/or acquisitions. We believe there are opportunities to acquire smaller, regional competitors that perform similar services to us, but do not have the national market access, a network of third party payor contracts or operating economies of scale that we currently enjoy. We also plan to leverage our extensive networks of oncology practices and insurers by distributing complementary products and introducing key new services.
We face risks that other competitors can provide the same services as us. Those risks are currently mitigated by our existing third party payor contracts and economies of scale, which allow for predictable reimbursement and less costly purchase and management of the pumps, respectively. Additionally, we have already established a long standing relationship as a provider of pumps to over 1,300 oncology practices in the United States. We believe that there are competitive barriers to entry against other suppliers with respect to these oncology practices because we have an established national presence and third party payor contracts in place covering approximately 195 million third party payor lives (i.e., persons enrolled in various managed care plans or commercial insurance carriers such as health maintenance organizations and preferred provider organizations) increasing the likelihood that we participate in the insurance networks of patients to whom physicians wish to refer an ambulatory infusion pump provider. Moreover, we have an available inventory of approximately 21,000 active ambulatory infusion pumps, which may allow us to be more responsive to the needs of physicians and patients than a new market entrant. We do not perform any research and development.
First Biomedical
On June 15, 2010, we acquired all of the issued and outstanding stock of First Biomedical, Inc (First Biomedical) pursuant to a Stock Purchase Agreement with the stockholders of First Biomedical.
First Biomedical sells, rents, services and repairs new and pre-owned infusion pumps and other medical equipment. It also sells a variety of primary and secondary tubing, cassettes, catheters and other disposable items that are utilized with infusion pumps. Headquartered in Olathe, Kansas, with additional facilities in California and Toronto, First Biomedical is a leading provider to alternate site healthcare facilities and hospitals in the United States and Canada. The acquisition of First Biomedical has allowed us to expand our offerings to existing customers with the addition of biomedical service and repair, while simultaneously bolstering the growth of infusion pump sales within our existing and potential future markets.
First Biomedical’s results of operations are included in our consolidated statements of operations from the date of acquisition.
Continuous Infusion Therapy
Continuous infusion of chemotherapy involves the gradual administration of a drug via a small, lightweight, portable electronic infusion pump over a prolonged period of time, defined as greater than 8 hours, and up to 24 hours daily. A cancer patient can receive his or her medicine anywhere from 1 to 30 days per month depending
3
on the chemotherapy regimen that is most appropriate to that individual’s health status and disease state. This may be followed by periods of rest and then repeated cycles with treatment goals of progression free disease survival. This drug administration method has replaced intravenous push or bolus administration in specific circumstances. The advantages of slow continuous low doses of certain drugs are well documented. Clinical studies support the use of continuous infusion chemotherapy for decreased toxicity without loss of anti-tumor efficacy. The 2009/2010 National Comprehensive Cancer Network (NCCN) Guidelines recommend the use of continuous infusion for treatment of numerous cancer diagnoses. We believe that the growth of continuous infusion therapy is driven by three factors: evidence of improved clinical outcomes; lower toxicity and side effects; and a favorable reimbursement environment.
| • | In the past decade, significant progress has been made in the treatment of colorectal cancer due to advances in surgery, radiotherapy and chemotherapy. In the late 1990s, medical researchers discovered that the delivery method of the drug (or schedule) was a key component to drug availability, efficacy and tolerability. Schedule dependant anti-tumor activity and toxicity has resulted in continuous infusion 5-Fluorouracil being adopted as the standard of care. In 2000, the FDA approved Camptosar (the trade name for the generic chemotherapy drug Irinotecan), a drug developed by Pfizer, for first-line therapy in combination with 5-Fluorouracil for the treatment of colorectal cancer. In 2002, the FDA approved Eloxatin (the trade name for the generic chemotherapy drug Oxaliplatin), a drug developed by Sanofi-Aventis, for use in combination with continuous infusion 5-Fluorouracil for the treatment of colorectal cancer. FOLFIRI, the chemotherapy protocol which includes Camptosar in combination with continuous infusion 5-Fluorouracil and the drug Leucovorin, and FOLFOX, the chemotherapy protocol which includes Eloxatin in combination with continuous infusion 5-Fluorouracil and Leucovorin, have resulted in significantly improved overall survival rates for colorectal cancer patients at various stages of the disease state. We believe that Sanofi-Aventis and Pfizer have each dedicated significant resources to educating physicians and promoting the use of FOLFOX and FOLFIRI. Simultaneously, the NCCN has established these regimens as the standards of care for the treatment of colorectal cancer. |
| • | The use of continuous infusion has been demonstrated to decrease or alter the toxicity of a number of cytotoxic, or cell killing agents. Higher doses of drugs can be infused over longer periods of time, leading to improved tolerance and decreased toxicity. For example, the cardiotoxicity (heart muscle damage) of the chemotherapy drug Doxorubicin is decreased by schedules of administration (The Chemotherapy Source Book, Perry, M.C.). Nausea, vomiting, diarrhea and decreased white blood cell and platelet counts are all affected by duration of delivery. Continuous infusion can lead to improved tolerance and patient comfort while enhancing the patient’s ability to remain on the chemotherapy regimen. Additionally, the lower toxicity profile and resulting reduction in side effects enables patients undergoing continuous infusion therapy to continue a relatively normal lifestyle, which may include continuing to work, go shopping, and care for family members. We believe that the partnering of physician management and patient autonomy provide for the highest quality of care with the greatest patient satisfaction. |
| • | We believe that oncology practices have a heightened sensitivity to whether and how much they are reimbursed for services. Simultaneously, the Center for Medicare and Medicaid Services (CMS) and private insurers are increasingly focusing on evidenced based medicine to inform their reimbursement decisions — that is, aligning reimbursement with clinical outcomes and adherence to standards of care. Continuous infusion therapy is a main component of the standard of care for certain cancer types because clinical evidence demonstrates superior outcomes. Payors recognize this and it is reflected in favorable reimbursement for clinical services related to the delivery of this care. |
Services
Our core service is to provide oncology offices, infusion clinics and hospital out-patient chemotherapy clinics with ambulatory infusion pumps in addition to related supplies for patient use. We then directly bill and collect payment from payors and patients for the use of these pumps. We own approximately 21,000 ambulatory
4
infusion pumps which are dedicated to this service offering. At any given time, it is estimated that approximately 60% of the pumps are in the possession of patients. The remainder of the pumps is in transport for cleaning and calibration, or in oncology clinics as back-ups.
After a doctor determines that a patient is eligible for ambulatory infusion pump therapy, the doctor arranges for the patient to receive an infusion pump and provides the necessary chemotherapy drugs. The oncologist and nursing staff train the patient in the use of the pump and initiate service. The physician bills insurers, Medicare, Medicaid, third party payor companies or patients (collectively, “payors”) for the physician’s professional services associated with initiating and supervising the infusion pump administration, as well as the supply of drugs. We directly bill payors for the use of the pump and related disposable supplies. We have contracts with more than 200 payors that cover approximately 195 million third party payor lives. Billing to payors requires coordination with patients and physicians who initiate the service, as physicians’ offices must provide us with appropriate paperwork (patient’s insurance information, physician’s order and an acknowledgement of benefits that shows receipt of equipment by the patient) in order for us to bill the payors.
In addition to providing high quality and convenient care, we believe that our business offers significant economic benefits for patients, providers and payors.
| • | We provide patients with 24-hour by 7 days (24x7) service and support. We employ oncology and intravenous certified registered nurses trained on ambulatory infusion pump equipment who staff our 24x7 hotline to address questions that patients may have about their pump treatment, the infusion pumps or other medical or technical questions related to the pumps. |
| • | Physicians use our services to outsource the capital commitment, pump service, maintenance and billing and administrative burdens associated with pump ownership. Our service also allows the doctor to continue a direct relationship with the patient and to receive professional service fees for setting up the treatment and administering the drugs. |
| • | We believe our services are attractive to payors because they are generally less expensive than hospitalization or home care. |
Other services we offer include the sales, rental and leasing of pole mounted and ambulatory infusion pumps to oncology practices, hospitals and other clinical settings. We own a fleet of approximately 14,000 new and used pole mounted and ambulatory pumps, representing over 70 makes and models of equipment which are dedicated to these services. These pumps are available for daily, weekly, monthly or annual rental periods as well as for sale or lease.
In addition to sales, rental and leasing services, the company also provides biomedical maintenance, repair and certification services for the devices we provide as well as for devices owned by customers but not acquired through InfuSystem. We operate pump service and repair Centers of Excellence across the United States and Canada and employ a staff of highly trained technicians to provide these services.
Relationships with Physician Offices
We have business relationships with clinical oncologists in more than 1,300 practices. Though this represents a substantial portion of the oncologists in the United States, we believe we can continue to expand our network to further penetrate the oncology market. Based on our high retention rates and the positive results of our professional customer satisfaction research, we believe our relationships with physician offices are strong.
We believe that, in general, we do not compete directly with hospitals and physician offices to treat patients. Rather, by providing products and services to hospitals and physician offices and other care facilities and providers, we believe that we assist other providers in meeting increasing patient demand and manage institutional constraints on capital and manpower due to the nature of limited resources in hospitals and physician offices.
5
Sales and Marketing
We employ a sales team of approximately 39 salespersons to coordinate our sales and marketing activities. Our efforts are directed primarily at physician’s offices, infusion clinics, hospital outpatient chemotherapy clinics and other enterprises serving patients who receive continuous infusions.
Employees
As of December 31, 2010, we had 180 employees, including 167 full-time employees and 13 part-time employees. None of our employees are unionized.
Company Officers
Sean McDevitt, Chairman and Chief Executive Officer
Mr. McDevitt has served as the Company’s Chairman of the Board since April 2005 and as Chief Executive Officer since September 2009. Mr. McDevitt is a founding principal, and since 2007 has been a Managing Director of Maren Group, an investment banking firm which provides mergers and acquisitions advisory services in the healthcare and technology sectors. Prior to joining Maren Group, Mr. McDevitt was a Managing Director of FTN Midwest Securities Corp. from September 2004 to January 2007. In 1999, Mr. McDevitt co-founded Alterity Partners, a boutique investment bank which provided capital markets and merger and acquisition advisory services to high growth companies. Alterity Partners was acquired by FTN Midwest Securities Corp. in September 2004. Mr. McDevitt was formerly a senior investment banker at Goldman Sachs & Company, from 1995 through 1999 where he led deal teams in a variety of technology and healthcare/biopharmaceutical transactions, including mergers and acquisitions, divestitures and initial public offerings. Prior to Goldman Sachs & Company, Mr. McDevitt worked in sales and marketing at Pfizer Inc. from 1991 until 1994. He was a Captain in the U.S. Army Rangers and was decorated for combat in the Panama invasion. He is a member of the Council on Foreign Relations. Mr. McDevitt received his B.S. in Computer Science and Electrical Engineering from the U.S. Military Academy at West Point and an M.B.A. from Harvard Business School.
James M. Froisland, Chief Financial Officer
Mr. Froisland has served as the Company’s Chief Financial Officer since December 2010. Prior to joining InfuSystem, from 2006 to 2010, Mr. Froisland served as Senior Vice President, Chief Financial Officer, Chief Information Officer and Corporate Secretary for Material Sciences Corporation (NASDAQ:MASC). Prior to this role, Mr. Froisland served as Senior Vice President, Chief Financial Officer and Chief Information Officer for InteliStaf Healthcare, Inc. and has held a variety of c-level and senior financial and information technology positions at Burns International Services Corporation, Anixter International Inc., Budget Rent A Car Corporation, Allsteel Inc., and The Pillsbury Company. Mr. Froisland started his career with KPMG, LLP and is a Certified Public Accountant. Mr. Froisland has an MBA, in Management Information Systems from the Carlson School of Management, University of Minnesota, and a BA, in Math and Accounting, from Luther College. Mr. Froisland also serves on the Board of Directors and Audit Committee for Westell Technologies, Inc. (NASDAQ:WSTL).
Material Suppliers
We supply a wide variety of pumps and associated equipment, as well as disposables and ancillary supplies. The majority of our pumps are electronic ambulatory pumps purchased from the following manufacturers, each of which is material and supplies more than 10% of the ambulatory pumps purchased by us: Smiths Medical, Inc.; Hospira Worldwide, Inc.; and WalkMed Infusion, LLC (formerly known as McKinley Medical, LLC). There are no supply agreements in place with any of the suppliers. All purchases are handled pursuant to pricing agreements, which contain no material terms other than prices that are subject to change by the manufacturer.
Seasonality
Our business is not subject to seasonality.
6
Environmental Laws
We are required to comply with applicable environmental laws regulating the disposal of cleaning agents used in the process of cleaning our ambulatory infusion pumps, as well as the disposal of sharps and blood products used in connection with the pumps. We do not believe that compliance with such laws has a material effect on our business.
Significant Customers
We have sought to establish contracts with as many third party payor organizations as commercially practicable, in an effort to ensure that reimbursement is not a significant obstacle for providers who recommend continuous infusion therapy and wish to utilize our services. A third party payor organization is a health care payor or a group of medical services payors that contracts to provide a wide variety of healthcare services to enrolled members through participating providers such as us. A payor is any entity that pays on behalf of a member patient.
We currently have contracts with more than 200 third party payor plans that cover approximately 195 million lives. Material terms of contracts with third party payor organizations are typically a set fee or rate, or discount from billed charges for equipment provided. These contracts generally provide for a term of one year, with automatic one-year renewals, unless we or the contracted payor do not wish to renew. Our largest contracted payor is Medicare, which accounted for approximately 31% of our gross billings for ambulatory infusion pump services for the year ended December 31, 2010. Our contracts with various individual Blue Cross/Blue Shield affiliates in the aggregate accounted for approximately 23% of our gross billings for ambulatory infusion pump services for the year ended December 31, 2010. We also contract with various other third party payor organizations, commercial Medicare replacement plans, self insured plans and numerous other insurance carriers. No individual payor, other than Medicare and the Blue Cross/Blue Shield entities, accounts for greater than approximately 6% of our ambulatory infusion pump services gross billings.
Competitors
We believe that our competition is primarily composed of regional providers, hospital-owned durable medical equipment (DME) providers, physician providers and home care infusion providers. An estimate of the number of competitors is not known or reasonably available, due to the wide variety in type and size of the market participants described below. We are not aware of any industry reports with respect to the competitive market described below. The description of market segments and business activities within those market segments is based on our experiences in the industry.
| • | Regional Providers: Regional DME providers act as distributors for a variety of medical products. We believe regional DME provider sales forces generally consist of a relatively small number of salespeople, usually covering several states. Regional DME providers tend to carry a limited selection of infusion pumps and their salespeople generally have limited resources. Regional DME providers usually do not have 24x7 nursing services. We believe that regional DME providers have relatively few third party payor contracts, which may prevent these providers from being paid at acceptable levels and may also result in higher out-of-pocket costs for patients. |
| • | Hospital-owned DME Providers: Many hospitals have in-house DME providers to supply basic equipment. In general, however, these providers have limited capital and tend to stock a small inventory of infusion pumps. We believe that hospital-owned providers have limited ability to grow because of restricted patient populations. Growth from outside of the hospital may pose a challenge because hospitals typically will not provide referrals to competitors, instead preferring to offer patients a choice of non-hospital-affiliated DME providers. |
| • | Physician Providers: A limited number of physicians maintain an inventory of their own infusion pumps and provide them to patients for a fee. However, we believe that pump utilization in this area |
7
| tends to be low and the costs associated with ongoing supplies, preventative maintenance and repairs can be relatively high. Moreover, we believe that a high percentage of DME claims by doctors are rejected by payors upon first submission, requiring a physician’s staff to spend significant time and effort to resubmit claims and receive payment for treatment. The numerous service and technical questions from patients may present another significant cost to a physician provider’s staff. |
| • | Home Care Infusion Providers: Home care infusion providers provide chemotherapy drugs and services to allow for in-home patient treatment. We believe that home care infusion treatment can be very costly and that many patients do not carry insurance coverage that covers home-based infusion services, resulting in larger out-of-pocket costs. Because home care treatments may take as long as six months, these costs can be high and can result in higher patient co-payments. We believe that home care providers may also be reluctant to offer 24x7 coverage or additional patient visits, due to capped fees. |
Regulation of Our Business
Our business is subject to certain regulations. Specifically, as a Medicare supplier of DME and related supplies, we must comply with DMEPOS Supplier Standards established by the Health Care Financing Administration regulating Medicare suppliers of DME and prosthetics, orthotics and supplies (DMEPOS). The DMEPOS Supplier Standards consist of 26 requirements that must be met in order for a DMEPOS supplier to be eligible to receive payment for a Medicare-covered item. Some of the more significant DMEPOS Supplier Standards require us to (i) advise Medicare beneficiaries of their option to purchase certain equipment, (ii) honor all warranties under state law and not charge Medicare beneficiaries for the repair or replacement of equipment or for services covered under warranty, (iii) permit agents of the Centers for Medicare and Medicaid Services to conduct on-site inspections to ascertain compliance with the DMEPOS Supplier Standards, (iv) maintain liability insurance in prescribed amounts, (v) refrain from contacting Medicare beneficiaries by telephone, except in certain limited circumstances, (vi) answer questions and respond to complaints of beneficiaries regarding the supplied equipment, (vii) disclose the DMEPOS Supplier Standards to each Medicare beneficiary to whom we supply equipment, (viii) maintain a complaint resolution procedure and record certain information regarding each complaint, (ix) maintain accreditation from a CMS approved accreditation organization and, (x) meet the surety bond requirements specified in 42 C.F.R. 424.57.
We are also subject to the provisions of the Health Insurance Portability and Accountability Act of 1996 (HIPAA) which are designed to protect the security and confidentiality of certain patient health information. Under HIPAA, we must provide patients access to certain records and must notify patients of our use of personal medical information and patient privacy rights. Moreover, HIPAA sets limits on how we may use individually identifiable health information and prohibits the use of patient information for marketing purposes. The adoption of the American Recovery and Reinvestment Act of 2009 (ARRA) includes a new breach notification requirement that applies to breaches of unsecured health information occurring on or after September 23, 2009.
We are subject to regulation in the various states in which we operate. We believe we are in compliance with all such regulation.
The healthcare industry is undergoing fundamental changes resulting from political, economic and regulatory influences. In the U.S., comprehensive programs are under consideration that seek to, among other things, increase access to healthcare for the uninsured and control the escalation of healthcare expenditures within the economy. On March 23, 2010, healthcare reform legislation (the “Healthcare Legislation”) was approved by Congress and has been signed into law. This legislation has only recently been enacted and requires the adoption of implementing regulations, which may impact our business.
Available Information
Our Internet address is www.infusystem.com. On this Web site, we post the following filings as soon as reasonably practicable after they are electronically filed with or furnished to the U.S. Securities and Exchange
8
Commission (the SEC): our Annual Reports on Form 10-K; our Quarterly Reports on Form 10-Q; our Current Reports on Form 8-K; our proxy statements related to our annual stockholders’ meetings; and any amendments to those reports or statements. All such filings are available on our Web site free of charge. The content on our Web site is not incorporated by reference into this Annual Report on Form 10-K unless expressly noted.
| Item 1A. | Risk Factors. |
An investment in our securities involves a high degree of risk. You should consider carefully all of the material risks described below, together with the other information contained in this Annual Report on Form 10-K. If any of the following events occur, our business, financial condition, results of operations and cash flows may be materially adversely affected.
RISK FACTORS RELATING TO OUR BUSINESS AND THE INDUSTRY IN WHICH WE OPERATE.
We are dependent on our Medicare Supplier Number.
We are required to have a Medicare Supplier Number in order to bill Medicare for services provided to Medicare patients. Furthermore, all third party and Medicaid contracts require us to have a Medicare Supplier Number. In addition, we are required to comply with Medicare Supplier Standards in order to maintain such number. If we are unable to comply with the relevant standards, we could lose our Medicare Supplier Number. The loss of such identification number for any reason would prevent us from billing Medicare for patients who rely on Medicare to pay their medical expenses and, as a result, we would experience a decrease in our revenues. Without such a number, we would be unable to continue our various third party and Medicaid contracts. A significant portion of our revenue is dependent upon our Medicare Supplier Number.
The Center for Medicare and Medicaid Services (CMS) has issued a ruling that all durable medical equipment (“DME”) providers must be accredited by a recognized accrediting entity by September 30, 2009. On February 17, 2009, we received accreditation from Community Health Accreditation Program (CHAP), thus meeting this CMS requirement. If we lost our accredited status, our financial condition, revenues and results of operations would be materially and adversely affected.
Changes in third-party reimbursement rates may adversely impact our revenues.
Our revenues are substantially dependent on third-party reimbursement. We are paid directly by private insurers and governmental agencies, often on a fixed fee basis, for continuous infusion equipment and related disposable supplies provided to patients. If the average fees allowable by private insurers or governmental agencies were reduced, the negative impact on revenues could have a material adverse effect on our financial condition, results of operations and cash flows. Also, if amounts owed to us by patients and insurers are reduced or not paid on a timely basis, we may be required to increase our bad debt expense and/or decrease our revenues.
Any change in the overall healthcare reimbursement system may adversely impact our business.
Changes in the healthcare reimbursement system often create financial incentives and disincentives that encourage or discourage the use of a particular type of product, therapy or clinical procedure. Market acceptance of continuous infusion therapy may be adversely affected by changes or trends within the healthcare reimbursement system. Changes to the health care reimbursement system that favor other technologies or treatment regimens that reduce reimbursements to providers or treatment facilities that use our services, may adversely affect our ability to market our services profitably.
9
Our success is impacted by the availability of the chemotherapy drugs that are used in our continuous infusion pump systems.
We primarily derive our revenue from the rental of ambulatory infusion pumps to oncology patients through physicians’ offices and chemotherapy clinics. A shortage in the availability of chemotherapy drugs that are used in the continuous infusion pump system could have a material adverse effect on our financial condition, results of operations and cash flows.
If future clinical studies demonstrate that oral medications are as effective as or more effective than continuous infusion therapy, our business could be adversely affected.
Numerous clinical trials are currently ongoing, evaluating and comparing the therapeutic benefits of current continuous infusion-based regimens with various oral medication regimens. If these clinical trials demonstrate that oral medications provide equal or greater therapeutic benefits and/or demonstrate reduced side effects compared to prior oral medication regimens, our revenues and overall business could be materially and adversely affected. Additionally, if new oral medications are introduced to the market that are superior to existing oral therapies, physicians’ willingness to prescribe continuous infusion-based regimens could decline, which would adversely affect our financial condition, results of operations and cash flows.
Global financial conditions may negatively impact our business, results of operations, financial condition and/or liquidity.
The recent global financial crisis affecting the banking system and financial markets, as well as the uncertainty in global economic conditions, have resulted in a significant tightening of credit markets, a low level of liquidity in financial markets and reduced corporate profits and capital spending. As a result, our customers (i.e., patients and payors) may face issues gaining timely access to sufficient credit, which could result in an impairment of their ability to make timely payments to us. In addition, the current global financial crisis could also adversely impact our suppliers’ ability to provide us with materials and components, either of which may negatively impact our financial condition, results of operations and cash flows. The financial crisis could also adversely impact our ability to access the financial markets.
Although we maintain allowances for doubtful accounts for estimated losses resulting from the inability of our customers to make required payments and such losses have historically been within our expectations and the provisions established, we cannot guarantee that we will continue to experience the same loss rates that we have in the past, especially given the current turmoil of the worldwide economy.
State licensure laws for durable medical equipment, or DME, suppliers are subject to change. If we fail to comply with any state’s laws, we will be unable to operate as a DME supplier in such state and our business operations will be adversely affected.
As a DME supplier operating in all 50 states of the United States, we are subject to each state’s licensure laws regulating DME suppliers. State licensure laws for DME suppliers are subject to change and we must ensure that we are continually in compliance with the laws of all 50 states. In the event that we fail to comply with any state’s laws governing the licensing of DME suppliers, we will be unable to operate as a DME supplier in such state until we regain compliance. We may also be subject to certain fines and/or penalties and our business operations could be adversely affected.
Our growth strategy includes expanding into treatment for cancers other than colorectal. There can be no assurance that continuous infusion-based regimens for these other cancers will become standards of care for large numbers of patients or that we will be successful in penetrating these different markets.
An aspect of our growth strategy is to expand into the treatment of other cancers, such as head, neck and gastric. Currently, relatively small percentages of these patients are treated with regimens that include continuous infusion therapy. That population will expand only if clinical trial results for new drugs and new combinations of
10
drugs demonstrate superior outcomes for regimens that include continuous infusion therapy relative to alternatives. No assurances can be given that these new drugs and drug combinations will be approved or will prove superior to oral medication or other treatment alternatives. In addition, no assurances can be given that we will be able to penetrate successfully any new markets that may develop in the future or manage the growth in additional resources that would be required.
The industry in which we operate is intensely competitive and changes rapidly. If we are unable to successfully compete with our competitors, our business operations may suffer.
The drug infusion industry is highly competitive. Some of our competitors and potential competitors have significantly greater resources than we do for research and development, marketing and sales. As a result, they may be better able to compete for market share, even in areas in which our services may be superior. The industry is subject to technological changes and such changes may put our current fleet of pumps at a competitive disadvantage. If we are unable to effectively compete in our market, our financial condition, results of operations and cash flows may materially suffer.
Our industry is dependent on regulatory guidelines that affect our billing practices. If our competitors do not comply with these regulatory guidelines, our business could be adversely affected.
Aggressive competitors may not fully comply with rules pertaining to documentation required by CMS and other payors for patient billing. Competitors, who don’t meet the same standards of compliance that we do with regards to billing regulations, can put us at a potential competitive disadvantage. We are a participating provider with Medicare and under contract with more than 200 additional insurance plans, all of which have very stringent guidelines. If our competitors do not comply with these regulatory guidelines, our business could be adversely affected.
We rely on independent suppliers for our products. Any delay or disruption in the supply of products, particularly our supply of electronic ambulatory pumps, may negatively impact our operations.
Our infusion pumps are obtained from outside vendors. The majority of our new pumps are electronic ambulatory infusion pumps which are supplied to us by three major suppliers: Smiths Medical, Inc.; Hospira Worldwide, Inc.; and WalkMed Infusion, LLC (formerly known as McKinley Medical, LLC). The loss or disruption of our relationships with outside vendors could subject us to substantial delays in the delivery of pumps to customers. Significant delays in the delivery of pumps could result in possible cancellation of orders and the loss of customers. Our inability to provide pumps to meet delivery schedules could have a material adverse effect on our reputation in the industry, as well as our financial condition, results of operations and cash flows.
Although we do not manufacture the products we distribute, if one of the products distributed by us proves to be defective or is misused by a health care practitioner or patient, we may be subject to liability that could adversely affect our financial condition and results of operations.
Although we do not manufacture the pumps that we distribute, a defect in the design or manufacture of a pump distributed by us, or a failure of pumps distributed by us to perform for the use specified, could have a material adverse effect on our reputation in the industry and subject us to claims of liability for injuries and otherwise. Misuse of the pumps distributed by us by a practitioner or patient that results in injury could similarly subject us to liability. Any substantial underinsured loss could have a material adverse effect on our financial condition, results of operations and cash flows. Furthermore, any impairment of our reputation could have a material adverse effect on our revenues and prospects for future business.
11
Unexpected costs or delays in integrating acquisitions could adversely affect our financial results.
During the year the Company acquired all of the outstanding stock of First Biomedical, and plans to make additional acquisitions going forward . As a result, we must devote significant management attention and resources to integrating the business practices and operations . We may encounter difficulties that could harm the businesses, adversely affect our financial condition, and cause our stock price to decline, including the following:
| • | We may have difficulty or experience delays in integrating the business and operations; |
| • | We may have difficulty maintaining employee morale and retaining key managers and other employees as we take steps to combine the personnel and business cultures of separate organizations into one, and to eliminate duplicate positions and functions; and |
| • | We may have difficulty preserving important relationships with others, such as strategic partners, customers, and suppliers, who may delay or defer decisions on agreements with us, or seek to change existing agreements with us, because of the acquisition. |
The integration process may divert the attention of our officers and management from day-to-day operations and disrupt our business, particularly if we encounter these types of difficulties. The failure of the combined company to meet the challenges involved in the integration process could cause an interruption of or a loss of momentum in the activities of the combined company and could seriously harm our results of operations.
Even if the operations are integrated successfully, the combined company may not fully realize the expected benefits of the transaction, including the synergies, cost savings or growth opportunities, whether within the anticipated time frame, or anytime in the future.
We intend to actively pursue opportunities for the further expansion of our business through strategic alliances, joint ventures and/or acquisitions. Future strategic alliances, joint ventures and/or acquisitions may require significant resources and/or result in significant unanticipated costs or liabilities to us.
Over the next two to three years we intend to actively pursue opportunities for the further expansion of our business through strategic alliances, joint ventures and/or acquisitions. Any future strategic alliances, joint ventures or acquisitions will depend on our ability to identify suitable partners or acquisition candidates, as the case may be, negotiate acceptable terms for such transactions and obtain financing, if necessary. We also face competition for suitable acquisition candidates which may increase our costs. Acquisitions or other investments require significant managerial attention, which may be diverted from our other operations. Any future acquisitions of businesses could also expose us to unanticipated liabilities.
If we engage in strategic acquisitions, we may experience significant costs and difficulty in assimilating operations or personnel, which could threaten our future growth.
If we make any acquisitions, we could have difficulty assimilating operations, technologies and products or integrating or retaining personnel of acquired companies. In addition, acquisitions may involve entering markets in which we have no or limited direct prior experience. The occurrence of any one or more of these factors could disrupt our ongoing business, distract our management and employees and increase our expenses. In addition, pursuing acquisition opportunities could divert our management’s attention from our ongoing business operations and result in decreased operating performance. Moreover, our profitability may suffer because of acquisition-related costs or amortization of intangible assets. Furthermore, we may have to incur debt or issue equity securities in future acquisitions. The issuance of equity securities would dilute our existing stockholders.
Covenants in our debt agreements restrict our business.
The credit agreement that governs our credit facility with Bank of America, N.A. and KeyBank National Association contains, and the agreements that govern our future indebtedness may contain, covenants that restrict our ability to and the ability of our subsidiaries to, among other things:
| • | create, incur, assume or suffer to exist any lien upon any of our property, assets or revenues; |
12
| • | make certain investments; |
| • | create, incur, assume or suffer to exist any indebtedness; |
| • | merge, dissolve, liquidate, consolidate all or substantially all of our assets; |
| • | make any disposition or enter into any agreement to make any disposition; and |
| • | declare or make, directly or indirectly, any dividend or other restricted payment, or incur any obligation (contingent or otherwise) to do so. |
Recently adopted healthcare reform legislation may adversely affect our business.
The healthcare industry is undergoing fundamental changes resulting from political, economic and regulatory influences. In the U.S., comprehensive programs are under consideration that seek to, among other things, increase access to healthcare for the uninsured and control the escalation of healthcare expenditures within the economy. On March 23, 2010, healthcare reform legislation (the “Healthcare Legislation”) was approved by Congress and has been signed into law. This legislation has only recently been enacted and requires the adoption of implementing regulations, which may impact our business. The Healthcare Legislation could have a material adverse effect on our business, financial condition and results of operations.
If we fail to comply with applicable healthcare regulations, we could face substantial penalties and our business, operations and financial condition could be adversely affected.
Certain federal and state healthcare laws and regulations pertaining to fraud and abuse and patients’ rights may be applicable to our business. We may be subject to healthcare fraud and abuse regulation and patient privacy regulation by both the federal government and the states in which we conduct our business. The laws that may affect our ability to operate include:
| • | the federal healthcare program Anti-Kickback Statute, which prohibits, among other things, soliciting, receiving or providing remuneration, directly or indirectly, to induce (i) the referral of an individual, for an item or service, or (ii) the purchasing or ordering of a good or service, for which payment may be made under federal healthcare programs such as the Medicare and Medicaid programs; |
| • | federal false claims laws which prohibit, among other things, knowingly presenting, or causing to be presented, claims for payment from Medicare, Medicaid, or other third-party payors that are false or fraudulent, and which may apply to entities like us that promote medical devices, provide medical device management services and may provide coding and billing advice to customers; |
| • | the federal Health Insurance Portability and Accountability Act of 1996, or HIPAA, which prohibits executing a scheme to defraud any healthcare benefit program or making false statements relating to healthcare matters and which also imposes certain requirements relating to the privacy, security and transmission of individually identifiable health information; and |
| • | state law equivalents of each of the above federal laws, such as anti-kickback and false claims laws that may apply to items or services reimbursed by any third-party payor, including commercial insurers, and state laws governing the privacy and security of health information in certain circumstances, many of which differ in significant ways from state to state and often are not preempted by HIPAA, thus complicating compliance efforts. |
Additionally, the compliance environment is changing, with more states, such as California and Massachusetts, mandating implementation of compliance programs, compliance with industry ethics codes, and spending limits, and other states, such as Vermont, Maine, and Minnesota, requiring reporting to state governments of gifts, compensation and other remuneration to physicians. Federal legislation, the Physician Payments Sunshine Act of 2009, has been proposed and is moving forward in Congress. This legislation would require disclosure to the federal government of payments to physicians. These laws all provide for penalties for
13
non-compliance. The shifting regulatory environment, along with the requirement to comply with multiple jurisdictions with different compliance and reporting requirements, increases the possibility that a company may run afoul of one or more laws.
If our operations are found to be in violation of any of the laws described above or any other governmental regulations that apply to us, we may be subject to penalties, including civil and criminal penalties, damages, fines and the curtailment or restructuring of our operations. Any penalties, damages, fines, curtailment or restructuring of our operations could adversely affect our ability to operate our business and our financial results. Any action against us for violation of these laws, even if we successfully defend against it, could cause us to incur significant legal expenses and divert our management’s attention from the operation of our business. Moreover, achieving and sustaining compliance with applicable federal and state privacy, security and fraud laws may prove costly.
We are dependent on key personnel, and the loss of any key employees or officers may have a materially adverse effect on our operations.
Our success is substantially dependent on the continued services of our executive officers and other key personnel who generally have extensive experience in our industry. Our future success also will depend in large part upon our ability to identify, attract and retain other highly qualified managerial, technical and sales and marketing personnel. Competition for these individuals is intense. The loss of the services of any key employees, or our failure to attract and retain other qualified and experienced personnel on acceptable terms, could have a material adverse effect on our business and results of operations.
The preparation of our financial statements in accordance with accounting principles generally accepted in the United States requires us to make estimates, judgments, and assumptions that may ultimately prove to be incorrect.
The accounting estimates and judgments that management must make in the ordinary course of business affect the reported amounts of assets and liabilities at the date of the financial statements and the reported amounts of revenue and expenses during the periods presented. If management misinterprets GAAP, subsequent adjustments resulting from errors could have a material adverse effect on our operating results for the period or periods in which the change is identified. Additionally, subsequent adjustments from errors could require us to restate our financial statements. Restating financial statements could result in a material decline in the price of our stock.
RISK FACTORS RELATING SPECIFICALLY TO OUR COMMON STOCK AND WARRANTS
The market price of our common stock has been, and is likely to remain, volatile and may decline in value.
The market price of our common stock has been and is likely to continue to be volatile. Market prices for securities of healthcare services companies, including ours, have historically been volatile, and the market has from time to time experienced significant price and volume fluctuations that appear unrelated to the operating performance of particular companies. The following factors, among others, can have a significant effect on the market price of our securities:
| • | announcements of technological innovations, new products, or clinical studies by others; |
| • | government regulation; |
| • | changes in the coverage or reimbursement rates of private insurers and governmental agencies; |
| • | announcements regarding new products or services or strategic alliances or acquisitions; |
| • | developments in patent or other proprietary rights; |
| • | the liquidity of the market for our common stock and warrants; |
14
| • | changes in health care policies in the United States or globally; |
| • | global financial conditions; and |
| • | comments by securities analysts and general market conditions. |
The realization of any risks described in these “Risk Factors” could also have a negative effect on the market price of our common stock and warrants.
We do not pay dividends and this may negatively affect the price of our stock.
Under the terms of our credit agreement with Bank of America, N.A. and KeyBank National Association, we are not permitted to pay dividends on our common stock and do not anticipate paying dividends on our common stock in the foreseeable future. The future price of our common stock may be adversely impacted because we do not pay dividends.
Future sales of our common stock may depress our stock price.
The market price of our common stock could decline as a result of sales of substantial amounts of our common stock in the public market, or the perception that these sales could occur. In addition to the shares of our common stock currently available for sale in the public market, shares of our common stock sold in past private placements (which include shares held by certain members of our board of directors) and the shares of common stock underlying our outstanding warrants are subject to registration rights. If the holders of these securities choose to exercise their registration rights, this would result in an increase in the number of shares of our common stock available for resale in the public market, which in turn could lead to a decrease in our stock price and a dilution of stockholders’ ownership interests. These factors could also make it more difficult for us to raise funds through future equity offerings.
Certain anti-takeover provisions in our amended and restated certificate of incorporation and bylaws and the Delaware General Corporation Law (the DGCL,), as well as our stockholders rights plan, may discourage, delay or prevent a change in control of our company and adversely affect the trading price of our common stock.
Our amended and restated certificate of incorporation and bylaws and the DGCL contain certain anti-takeover provisions which may discourage, delay or prevent a change in control of our company that our stockholders may consider favorable and, as a result, adversely affect the trading price of our common stock. Our amended and restated certificate of incorporation authorizes our board of directors to issue up to 1,000,000 shares of blank check preferred stock. Our amended and restated bylaws include provisions establishing advance notice procedures with respect to stockholder proposals and director nominations and permitting only stockholders holding at least a majority of our outstanding common stock to call a special meeting. Additionally, as a Delaware corporation, we are subject to section 203 of the DGCL, which, among other things, and subject to various exceptions, restricts certain business transactions between a corporation and a stockholder owning 15% or more of the corporation’s outstanding voting stock (“an interested stockholder”) for a period of three years from the date the stockholder becomes an interested stockholder.
In addition, our board of directors has adopted a stockholder rights plan. This plan would cause the substantial dilution of the holdings of any person that attempts to acquire us without the approval of our board of directors.
| Item 1B. | Unresolved Staff Comments. |
None.
15
| Item 2. | Properties. |
We do not own any real property. We lease office and warehouse space at the following locations:
| Madison Heights | MI | |
| New York | NY | |
| Bennington | VT | |
| Olathe | KS | |
| Santa Fe Springs | CA | |
| Mississauga | Ontario, Canada |
We believe that such office and warehouse space is suitable and adequate for our business.
| Item 3. | Legal Proceedings. |
We are involved in legal proceedings arising out of the ordinary course and conduct of our business, the outcomes of which are not determinable at this time. We have insurance policies covering such potential losses where such coverage is cost effective. In our opinion, any liability that might be incurred by us upon the resolution of these claims and lawsuits will not, in the aggregate, have a material adverse effect on our financial condition, results of operations or cash flows.
16
| Item 5. | Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities. |
Our common stock is currently traded on the NYSE Amex under the symbol INFU. Our warrants and units are currently traded on the OTC Bulletin Board under the symbols INHIU.OB and INHIW.OB, respectively. Prior to December 23, 2010, our common stock was traded on the OTC Bulletin Board under the symbol INHI.OB.
Each warrant entitles the holder to purchase from us one share of our common stock at an exercise price of $5.00. Our warrants will expire at 5:00 p.m., New York City time, on April 11, 2011, or earlier upon redemption.
The following tables set forth, for the calendar quarter indicated, the quarterly high and low bid information of our common stock, units and warrants, respectively, as reported on the NYSE Amex or the OTC Bulletin Board, as applicable. The quotations listed below reflect interdealer prices, without retail markup, markdown or commission and may not necessarily represent actual transactions.
Common Stock
| Quarter ended |
High | Low | ||||||
| December 31, 2010 |
$ | 2.70 | $ | 2.10 | ||||
| September 30, 2010 |
$ | 2.70 | $ | 2.05 | ||||
| June 30, 2010 |
$ | 2.70 | $ | 2.25 | ||||
| March 31, 2010 |
$ | 2.85 | $ | 2.10 | ||||
| December 31, 2009 |
$ | 3.00 | $ | 2.15 | ||||
| September 30, 2009 |
$ | 3.00 | $ | 2.15 | ||||
| June 30, 2009 |
$ | 3.25 | $ | 2.08 | ||||
| March 31, 2009 |
$ | 2.50 | $ | 1.52 | ||||
Units*
| Quarter ended |
High | Low | ||||||
| December 31, 2010 |
$ | 2.05 | $ | 2.05 | ||||
| September 30, 2010 |
$ | 1.50 | $ | 1.50 | ||||
| June 30, 2010 |
$ | 1.50 | $ | 1.50 | ||||
| March 31, 2010 |
$ | 2.45 | $ | 2.35 | ||||
| December 31, 2009 |
$ | 2.20 | $ | 2.10 | ||||
| September 30, 2009 |
$ | 2.10 | $ | 2.10 | ||||
| June 30, 2009 |
$ | 2.10 | $ | 2.10 | ||||
| March 31, 2009 |
$ | 2.10 | $ | 2.10 | ||||
| * | There are 1,650 units outstanding as of December 31, 2010 which are included within common stock in the consolidated financial statements. |
Warrants
| Quarter ended |
High | Low | ||||||
| December 31, 2010 |
$ | .04 | $ | .01 | ||||
| September 30, 2010 |
$ | .08 | $ | .02 | ||||
| June 30, 2010 |
$ | .10 | $ | .06 | ||||
| March 31, 2010 |
$ | .09 | $ | .053 | ||||
| December 31, 2009 |
$ | .10 | $ | .05 | ||||
| September 30, 2009 |
$ | .11 | $ | .05 | ||||
| June 30, 2009 |
$ | .12 | $ | .065 | ||||
| March 31, 2009 |
$ | .125 | $ | .05 | ||||
17
Holders of Common Equity
As of February 16, 2011, we had approximately 359 stockholders of record of our common stock. This does not include beneficial owners of our common stock, including Cede & Co., nominee of the Depository Trust Company.
Dividends
We have not paid any dividends on our common stock to date. The payment of dividends in the future will be contingent upon our revenues and earnings, if any, capital requirements and general financial condition. Under the terms of our credit agreement with Bank of America, N.A. and KeyBank National Association, we are not permitted to pay dividends. It is the present intention of our board of directors to retain all earnings, if any, for use in our business operations and, accordingly, our board of directors does not anticipate declaring any dividends in the foreseeable future.
Equity Compensation Plan Information
The following table provides information as of December 31, 2010 with respect to compensation plans, including individual compensation arrangements, under which our equity securities are authorized for issuance.
| Plan Category |
Number of securities to be issued upon exercise of outstanding options, warrants and rights |
Weighted-average exercise price of outstanding options, warrants and rights (2) |
Number of securities remaining available for future issuance under equity compensation plans (excluding securities reflected in column (a)) |
|||||||||
| (a) | (b) | (c) | ||||||||||
| Equity compensation plans approved by security holders (1) |
191,229 | $ | 2.66 | 68,437 | ||||||||
| Equity compensation plans not approved by security holders (3) |
2,112,500 | Not Applicable | Not Applicable | |||||||||
| Total |
2,303,729 | $ | 2.66 | 68,437 | ||||||||
| (1) | This amount includes 60,750 shares of common stock issuable upon the vesting of certain restricted stock awards (the “Restricted Stock Awards”) and 130,479 shares of common stock issuable upon the exercise of a vested stock option award (the “Stock Option”) made under the InfuSystem Holdings, Inc. 2007 Stock Incentive Plan (the “Plan”). This amount does not include 237,500 shares of common stock which vested under the terms of the Restricted Stock Awards during the year ended December 31, 2010. This amount also does not include 1,125,000 shares of common stock issuable upon the vesting of Restricted Stock Awards granted to directors in 2010, all of which vested prior to December 31, 2010. |
| (2) | Represents the exercise price of the Stock Option. |
| (3) | This amount includes 2,112,500 shares of common stock issuable upon the vesting of certain Restricted Stock Awards made outside of the Plan during the year ended December 31, 2010. This amount does not include 62,500 shares of common stock which vested under the terms of the Restricted Stock Awards during the year ended December 31, 2010. This amount also does not include 50,000 shares of common stock issuable upon the vesting of a Restricted Stock Award granted to a director in 2010, all of which vested prior to December 31, 2010. |
18
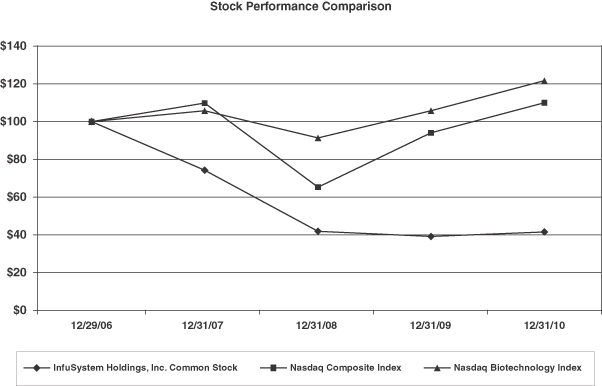
Stock Performance Graph
The graph set forth below compares the change in the our cumulative total stockholder return on our common stock between December 29, 2006 and December 31, 2010 with the cumulative total return of the NASDAQ Composite Index and the NASDAQ Biotechnology Index during the same period. This graph assumes the investment of $100 on December 29, 2006 in our common stock and each of the comparison groups and assumes reinvestment of dividends, if any. We have not paid any dividends on our common stock, and no dividends are included in the report of our performance. This graph is not “soliciting material,” is not deemed filed with the SEC and is not to be incorporated by reference in any of our filings under the Securities Act or the Exchange Act whether made before or after the date hereof and irrespective of any general incorporation language in any such filing.

| 12/29/06 | 12/31/07 | 12/31/08 | 12/31/09 | 12/31/10 | ||||||||||||||||
| InfuSystem Holdings, Inc. Common Stock |
$ | 100.00 | $ | 74.24 | $ | 42.04 | $ | 39.36 | $ | 41.68 | ||||||||||
| Nasdaq Composite Index |
$ | 100.00 | $ | 109.81 | $ | 65.29 | $ | 93.95 | $ | 109.84 | ||||||||||
| Nasdaq Biotechnology Index |
$ | 100.00 | $ | 105.71 | $ | 91.38 | $ | 105.68 | $ | 121.52 | ||||||||||
Recent Sales of Unregistered Securities
None.
Repurchases of Equity Securities
As previously announced, our board of directors has authorized a share repurchase program of up to $2 million of our outstanding common shares. The repurchase program will be funded by our available cash balance.
19
Stock repurchases may be made through open market transactions, negotiated purchases or otherwise, at times and in such amounts as our management deems to be appropriate. The timing and actual number of shares repurchased will depend on a variety of factors, including price, financing and regulatory requirements, as well as other market conditions. The program does not require us to repurchase any specific number of shares or to complete the program within a specific period of time.
The following table provides information about our purchased of common stock during the fourth quarter of the year ended December 31, 2010:
| (period) |
Total Number of Shares Purchased |
Average Price Paid per Share |
Total Number of Shares Purchased as Part of Publicly Announced Plans or Programs |
Approximate Dollar Value of Shares that May Yet Be Purchased Under the Plans or Programs |
||||||||||||
| October 1, 2010 — October 31, 2010 |
— | $ | — | — | $ | — | ||||||||||
| November 1, 2010 — November 30, 2010 |
9,574 | 2.45 | 9,574 | 1,976,000 | ||||||||||||
| December 1, 2010 — December 31, 2010 |
36,247 | 2.46 | 36,247 | 1,887,000 | ||||||||||||
| Total for fourth quarter of 2010 |
45,821 | $ | 2.46 | 45,821 | $ | 1,887,000 | ||||||||||
20
| Item 6. | Selected Financial Data. |
InfuSystem Holdings, Inc. and Subsidiaries
You should read the following selected financial data together with our financial statements and related notes included in Item 8 of this Annual Report on Form 10-K, and with “Management’s Discussion and Analysis of Financial Condition and Results of Operations” included in Item 7 of this Annual Report on Form 10-K. We have derived the statement of operations data for the years ended December 31, 2010, 2009 and 2008 and the balance sheet data as of December 31, 2010 and 2009 from our audited financial statements, which are included in Item 8 of this Annual Report on Form 10-K. Our historical results for any period are not necessarily indicative of results to be expected for any future period. The information for InfuSystem Holdings, Inc. for the year ended December 31, 2007 includes operations for InfuSystem from October 26, 2007 through December 31, 2007.
Statement of Operations Data (1)
| (in thousands, except per share data) |
Year Ended December 31, 2010 |
Year Ended December 31, 2009 |
Year Ended December 31, 2008 |
Year Ended December 31, 2007 |
||||||||||||
| Net revenues |
$ | 47,229 | $ | 38,964 | $ | 35,415 | $ | 6,582 | ||||||||
| Total operating expenses |
(48,167 | ) | (33,636 | ) | (30,629 | ) | (8,079 | ) | ||||||||
| Total other (loss) income |
(2,285 | ) | (3,577 | ) | 6,080 | (189 | ) | |||||||||
| Income tax (benefit) expense |
(1,371 | ) | (977 | ) | (907 | ) | (1,110 | ) | ||||||||
| Net (loss) income |
(1,852 | ) | 774 | 9,959 | (2,796 | ) | ||||||||||
| Net (loss) income per share — basic |
$ | (0.09 | ) | $ | 0.04 | $ | 0.56 | $ | (0.15 | ) | ||||||
| Net (loss) income per share — diluted |
$ | (0.09 | ) | $ | 0.04 | $ | 0.53 | $ | (0.15 | ) | ||||||
| Balance Sheet Data (at period end) (1) | ||||||||||||||||
| (in thousands) |
December 31, 2010 |
December 31, 2009 |
December 31, 2008 |
December 31, 2007 |
||||||||||||
| Total assets |
$ | 130,364 | $ | 114,690 | $ | 116,220 | $ | 116,426 | ||||||||
| Long-term debt, including current maturities |
32,197 | 24,141 | 30,669 | 32,294 | ||||||||||||
| Stockholders’ equity |
85,086 | 81,465 | 80,073 | 68,759 | ||||||||||||
| (1) | On October 25, 2007, we completed our acquisition of 100% of the issued and outstanding capital stock of InfuSystem from I-Flow pursuant to the terms of the Stock Purchase Agreement. InfuSystem’s results of operations are included in our Consolidated Statements of Operations from the date of the acquisition. For more information, see Note 3 “Acquisitions” to our Consolidated Financial Statements which are included in this Annual Report on Form 10-K. |
Predecessor InfuSystem
The statement of operations data for the period from January 1, 2007 to October 25, 2007 and fiscal year ended December 31, 2006 and the balance sheet data as of December 31, 2006 was derived from the audited financial statements of Predecessor InfuSystem, which are not included in this report.
Statement of Operations Data
| January 1, 2007 to October 25, 2007 |
Year Ended December 31, 2006 |
|||||||
| Net revenues |
$ | 25,001 | $ | 31,716 | ||||
| Cost of revenues |
6,702 | 8,455 | ||||||
| Total operating expenses |
15,673 | 15,091 | ||||||
| Income tax expense |
1,086 | 3,094 | ||||||
| Net income |
1,777 | 4,963 | ||||||
21
Balance Sheet Data (at period end)
| December 31, 2006 |
||||
| Total assets |
$ | 27,628 | ||
| Stockholders’ equity |
22,008 | |||
| Item 7. | Management’s Discussion and Analysis of Financial Condition and Results of Operations. |
Overview
We are the leading provider of infusion pumps and related services. We service hospitals, oncology practices and other alternate site healthcare providers. Headquartered in Madison Heights, Michigan, we deliver local, field-based customer support, and also operate Centers of Excellence in Michigan, Kansas, California, and Ontario, Canada.
We supply electronic ambulatory infusion pumps and associated disposable supply kits to oncology practices, infusion clinics and hospital outpatient chemotherapy clinics. These pumps and supplies are utilized primarily by colorectal cancer patients who receive a standard of care treatment that utilizes continuous chemotherapy infusions delivered via electronic ambulatory infusion pumps. We obtain an assignment of insurance benefits from the patient, bill the insurance company or patient accordingly, and collect payment. We provide pump management services for the pumps and associated disposable supply kits to over 1,300 oncology practices in the United States, and retain title to the pumps during this process.
We sell or rent new and pre-owned pole mounted and ambulatory infusion pumps to, and provide biomedical recertification, maintenance and repair services for, oncology practices as well as other alternate site settings including home care and home infusion providers, skilled nursing facilities, pain centers and others.
On June 15, 2010, we entered into a stock purchase agreement with the shareholders of First Biomedical, Inc. to acquire all of the issued and outstanding stock of First Biomedical and completed the acquisition for total consideration of $17.4 million. First Biomedical’s results of operations are included in our consolidated statements of operations from the acquisition date.
First Biomedical sells, rents, services and repairs new and pre-owned infusion pumps and other medical equipment. First Biomedical also sells a variety of primary and secondary tubing, cassettes, catheters and other disposable items that are utilized with infusion pumps. Headquartered in Olathe, KS, with additional facilities in California and Toronto, First Biomedical is a leading provider to alternate site healthcare facilities and hospitals in the United States and Canada.
InfuSystem Holdings, Inc. Results of Operations for the Year ended December 31, 2010 compared to the Year ended December 31, 2009
Revenues
Our revenue for the year ended December 31, 2010 was $47.2 million, a 21% increase compared to $39.0 million for the year ended December 31, 2009. The increase in revenues is primarily related to revenues generated by recently acquired First Biomedical, obtaining business at new customer facilities, as well as deeper penetration into existing customer facilities.
Gross Profit
Gross profit for the year ended December 31, 2010 was $33.5 million, an increase of 17% compared to $28.6 million in the prior year. It represented 71% of revenues in the current year compared to 73% in the prior
22
year. The decrease, as a percentage of revenues, is primarily related to higher pump depreciation and disposal costs, a higher mix of pump sales and services, including First Biomedical, as compared to third party billings, partially offset by lower supplies costs.
Provision for Doubtful Accounts
Provision for doubtful accounts for the year ended December 31, 2010 was $4.5 million, compared to $4.0 million for the year ended December 31, 2009. The provision for doubtful accounts remained consistent at 10% of revenues for the year ended December 31, 2010, compared to the year ended December 31, 2009.
Amortization of Intangible Assets
Amortization of intangible assets for the year ended December 31, 2010 was $2.3 million, a 28% increase compared to $1.8 million for the year ended December 31, 2009. The increase is primarily related to additional intangible assets associated with the acquisition of First Biomedical, as well as amortization of new software.
Selling and Marketing Expenses
For the year ended December 31, 2010, our selling and marketing expenses were $7.1 million compared to $5.3 million for the year ended December 31, 2009. Selling and marketing expenses during these periods consisted of sales salaries, commissions and associated fringe benefit and payroll-related items, marketing, share-based compensation, travel and entertainment and other miscellaneous expenses. The increase in expenses is primarily related to expenses incurred by recently acquired First Biomedical. As compared to the prior year, these expenses increased from 13% to 15% of revenues for the year ended December 31, 2010.
General and Administrative Expenses
During the year ended December 31, 2010, our general and administrative expenses were $20.6 million, compared to $12.2 million for the year ended December 31, 2009. The increase is primarily related to an increase in share-based compensation, expenses incurred at recently acquired First Biomedical, and costs associated with the acquisition of First Biomedical. General and administrative expenses during these periods consisted primarily of administrative personnel salaries, fringe benefits and payroll-related items, professional fees, share-based compensation, insurance and other miscellaneous expenses. General and administrative expenses have increased from 31% to 44% of revenues for the year ended December 31, 2010 compared to the same period in the prior year. The increase as a percentage of revenue is primarily related to an increase in share-based compensation expense.
Other Income and Expenses
During the year ended December 31, 2010, we recorded a gain on derivatives of $207 thousand, compared to a loss of $78 thousand during the year ended December 31, 2009. Included in the year ended December 31, 2010 gain was an unrealized gain from the change in fair value of our warrants, a realized loss recorded in connection with the warrant exchange, and a realized gain on the termination of our prior interest rate swap, whereas the year ended December 31, 2009 loss included an unrealized loss from the change in the fair value of our warrants and an unrealized gain from the change in the fair value of the interest rate swap that was in place at the time. For more information, refer to the discussion under “Summary of Significant Accounting Policies — Warrants and Derivative Financial Instruments” included in Note 2 and “Warrants and Derivative Financial Instruments” included in Note 6 to our Consolidated Financial Statements included in this Annual Report on Form 10-K.
During the year ended December 31, 2010, we recorded interest expense of $3.4 million, compared to $3.5 million for the year ended December 31, 2009. These amounts consist primarily of interest paid on our term
23
loans, cash payments associated with our terminated and new interest rate swaps, amortization of deferred debt issuance costs and interest expense on capital leases. The decrease is primarily related to a lower interest rate of the new term loan as well as a lower swap rate. These were offset by a one-time expensing of all of the remaining I-Flow deferred debt issuance costs and an increase in capital leases.
During the year ended December 31, 2010, we recorded income tax benefit of $1.4 million, compared to an expense of $977 thousand for the year ended December 31, 2009. The effective tax rate for the year ended December 31, 2010 was 47.21%, compared to 55.43% for the year ended December 31, 2009. The effective tax rate of 47.21% for the year ended December 31, 2010, as compared to the statutory rate of 34%, is primarily driven by permanent items including the current change in the valuation allowance on net deferred tax assets, the change in the net deferred tax liability on indefinite-lived goodwill and various state tax expenses. Refer to the discussion under “Summary of Significant Accounting Policies — Income Taxes” included in Note 2 and “Income Taxes” included in Note 8 to our Consolidated Financial Statements included in this Annual Report on Form 10-K.
Inflation
Management believes that there has been no material effect on our operations or financial condition as a result of inflation or changing prices of our ambulatory infusion pumps during the period from December 31, 2009 through December 31, 2010.
InfuSystem Holdings, Inc. Results of Operations for the Year ended December 31, 2009 compared to the Year ended December 31, 2008
Revenues
Our revenue is predominantly derived from our rental of ambulatory infusion pumps which are primarily used for continuous infusion of chemotherapy drugs for patients with colorectal cancer. Our revenue for the year ended December 31, 2009 was $39.0 million, an 11% improvement compared to $35.4 million for the year ended December 31, 2008. The increase in revenues is primarily due to obtaining business at new customer facilities, improved operational efficiency tools which led to successful billing of older or delayed documentation, as well as increased reimbursement.
Gross Profit
Gross profit for the year ended December 31, 2009 was $28.6 million, up 9% compared to $26.2 million for the year ended December 31, 2008. It represented 73% of revenues for the year ended December 31, 2009 compared to 74% for the year ended December 31, 2008. The decrease, as a percent of revenues, was primarily related to increased revenues, as well as higher pump repair and maintenance costs, offset by lower freight costs as compared to the prior period.
Amortization of Intangible Assets
Amortization of intangible assets for the year ended December 31, 2009 was $1.8 million, which was identical to the amount recognized for the year ended December 31, 2008. This represents the annual amortization expense associated with our Physician Relationships, which we amortize over 15 years.
Provision for doubtful accounts
Provision for doubtful accounts for the year ended December 31, 2009 was $4.0 million, compared to $3.2 million for the year ended December 31, 2008. The provision for doubtful accounts has increased slightly from 9% to 10% of revenues for the year ended December 31, 2009, compared to the year ended December 31, 2008. The increase, as a percentage of revenue, is directly related to a slight increase in the mix of billings directly to patients, as compared to billings to third-party payors.
24
Selling and Marketing Expenses
For the year ended December 31, 2009, our selling and marketing expenses were $5.3 million, compared to $4.7 million for the year ended December 31, 2008. Selling and marketing expenses during these periods consisted of sales salaries, commissions and associated fringe benefit and payroll-related items, travel and entertainment, marketing, share-based compensation, and other miscellaneous expenses. These expenses have remained fairly consistent as a percentage of revenues, at approximately 13% for the years ended December 31, 2009 and 2008.
General and Administrative Expenses
During the year ended December 31, 2009, our general and administrative expenses were $12.2 million, compared to $11.8 million for the year ended December 31, 2008. General and administrative expenses during these periods consisted primarily of administrative personnel, including management and officers’ salaries, fringe benefits and payroll-related items, professional fees, share-based compensation, insurance (including directors’ and officers’ insurance) and other miscellaneous expenses. The expenses in total have decreased slightly from 33% to 31% of revenues for the year ended December 31, 2009, compared to the year ended December 31, 2008. The decrease, as a percentage of revenues, for the year ended December 31, 2009 is primarily driven by a decrease in stock based compensation and a decrease in professional fees primarily related to significant efficiencies associated with the preparation and audit of our Annual Report on Form 10-K. These decreases were partially offset by the recognition of Steve Watkins’, our former CEO, compensation and benefits in accordance with his separation agreement.
Other Income and Expenses
During the year ended December 31, 2009, we recorded a loss on derivatives of $78 thousand, compared to a gain of $9.8 million during the year ended December 31, 2008. These amounts represent an unrealized (loss) gain which resulted from the change in the fair value of our warrants, combined with an unrealized gain (loss) resulting from the change in the fair value of our single interest rate swap.
During the year ended December 31, 2009, we recorded interest expense of $3.5 million, compared to $3.8 million for the year ended December 31, 2008. These amounts consist of interest paid to Kimberly-Clark (formerly I-Flow) on our term loan, the amortization of deferred debt issuance costs incurred in conjunction with the loan, expense associated with the interest rate swap and interest expense on capital leases for ambulatory pumps. The decrease is primarily the result of a decrease in interest expense on the term loan with Kimberly-Clark (formerly I-Flow). This was the result of a decrease in the outstanding balance due to significant principal payments made during the year ended December 31, 2009. This was partially offset by higher cash payments associated with our single interest rate swap, due to the LIBOR rate being significantly lower during the year ended December 31, 2009 as compared to the year ended December 31, 2008. The decrease was also partially offset by interest expense related to new capital leases that we entered into during the year to finance the purchase of ambulatory pumps.
During the year ended December 31, 2009, we recorded income tax expense of $977 thousand, compared to $907 thousand for the year ended December 31, 2008. The effective tax rate for the year ended December 31, 2009 was 55.43%, compared to 8.34% for the year ended December 31, 2008. The effective tax rate of 55.4% for the year ended December 31, 2009, as compared to the statutory rate of 34%, is primarily driven by permanent items including the current change in the valuation allowance on net deferred tax assets, the change in the net deferred tax liability on indefinite-lived goodwill and various state tax expenses.
Liquidity and Capital Resources
As of December 31, 2010 we had cash resources of $5.0 million compared to $7.8 million at December 31, 2009. The decrease in cash was primarily related to cash used for the acquisition of First Biomedical, partially offset by an increase in outstanding term debt and positive cash flows from operating activities.
25
Cash provided by operating activities for the year ended December 31, 2010 was $10.8 million, compared to cash provided by operating activities of $9.7 million for the year ended December 31, 2009. The increase is primarily attributable to higher revenues and earnings, not including non-cash items such as stock based compensation and depreciation.
Cash used in investing activities for the year ended December 31, 2010 was $19.1 million, compared to $4.6 million for the year ended December 31, 2009. The increase is primarily related to cash paid for the acquisition of First Biomedical, partially offset by lower purchases of infusion pumps, more extensive use of capital leases, as a percentage of acquisitions, to acquire such equipment, and the non-repeat of first half 2009 expenditures associated with both moving our office facilities and investments in customized software.
Cash provided by financing activities for the year ended December 31, 2010 was $5.5 million, compared to cash used in financing activities of $8.9 million for the year ended December 31, 2009. The increase is primarily related to an increase in outstanding term debt, partially offset by upfront costs associated with our new credit facilities and higher principal payments associated with capital leases.
Management believes the current funds, together with expected cash flows from ongoing operations as well as the $4.9 million available on the revolving credit facility from Bank of America referred to below, are sufficient to fund our current operations for at least the next 12 months.
On June 15, 2010, we entered into a credit facility with Bank of America, N.A. as Administrative Agent, and KeyBank National Association as Documentation Agent. The facility consists of a $30.0 million term loan and a $5.0 million revolving credit facility, both of which mature in June 2014. Interest on the term loan is payable at our choice of LIBOR plus 4.5%, or the Bank of America prime rate plus 3.5%. As of December 31, 2010, interest was payable at LIBOR plus 4.5%, which equaled approximately 4.76%.
Proceeds from the new term loan were used to repay the outstanding balance of our debt held by Kimberly-Clark (I-Flow), as well as contribute to the acquisition consideration for First Biomedical. As of December 31, 2010, the Company had a letter of credit in the amount of $81 thousand outstanding, leaving $4.9 million available on its revolving credit facility.
The Bank of America term loan is collateralized by substantially all of our assets and requires us to comply with covenants principally relating to satisfaction of a total leverage ratio, a fixed charge coverage ratio, and an annual limit on capital expenditures. As of December 31, 2010, we believe we were in compliance with all such covenants.
Contractual Obligations
As of December 31, 2010, future payments related to contractual obligations are as follows:
| Payment Due by Period (1) (2) | ||||||||||||||||||||
| Less than 1 Year |
1 to 3 Years |
3 to 5 Years |
More than 5 Years |
Total | ||||||||||||||||
| (Amounts in Thousands) | ||||||||||||||||||||
| Debt obligations |
$ | 4,500 | $ | 9,569 | $ | 14,625 | $ | — | $ | 28,694 | ||||||||||
| Capital Lease Obligations |
1,051 | 2,236 | 216 | — | 3,503 | |||||||||||||||
| Operating Lease Obligations |
481 | 596 | 395 | — | 1,472 | |||||||||||||||
| Total |
$ | 6,032 | $ | 12,401 | $ | 15,236 | $ | — | $ | 33,669 | ||||||||||
| (1) | The table above does not include any potential payout to Kimberly-Clark (formerly I-Flow) associated with the earn-out provision in the Stock Purchase Agreement. For more information, refer to the discussion under “Acquisitions” included in Note 3 to our Consolidated Financial Statements included in this Annual Report on Form 10-K. |
| (2) | The table above does not include any interest payments associated with our variable rate term debt. For more information, refer to the discussion under “Debt and other Long-term Obligations” included in Note 7 to our Consolidated Financial Statements included in this Annual Report on Form 10-K. |
26
Included in the operating lease obligations are future minimum lease payments as of December 31, 2010 under various lease agreements we have entered into for office space.
Contingent Liabilities
We do not have any contingent liabilities.
Off-Balance Sheet Arrangements
We do not have any material off-balance sheet arrangements.
Critical Accounting Policies and Estimates
The preparation of financial statements in conformity with GAAP requires management to make estimates, assumptions and judgments that affect the amounts reported in the financial statements, including the notes thereto. We consider critical accounting policies to be those that require more significant judgments and estimates in the preparation of our consolidated financial statements, including the following: revenue recognition, which includes contractual allowances; accounts receivable and allowance for doubtful accounts; warrants and derivative financial instruments; income taxes; and goodwill valuation. Management relies on historical experience and other assumptions believed to be reasonable in making its judgment and estimates. Actual results could differ materially from those estimates.
Management believes its application of accounting policies, and the estimates inherently required therein, are reasonable. These accounting policies and estimates are periodically reevaluated, and adjustments are made when facts and circumstances dictate a change.
Our accounting policies are more fully described under the heading “Summary of Significant Accounting Policies” in Note 2 to our Consolidated Financial Statements included in this Annual Report on Form 10-K. We believe the following critical accounting estimates are the most significant to the presentation of our financial statements and require the most difficult, subjective and complex judgments:
Revenue Recognition
The majority of our revenue is rental revenue in the oncology market. Revenues are recognized predominantly under fee for service arrangements through equipment that we rent to patients. We recognize revenue only when all of the following criteria are met: 1) persuasive evidence of an arrangement exists; 2) services have been rendered; 3) the price to the customer is fixed or determinable; and 4) collectability is reasonably assured. Persuasive evidence of an arrangement is determined to exist, and collectability is reasonably assured, when 1) we receive a physician’s written order and assignment of benefits, signed by the physician and patient, respectively, and 2) we have verified actual pump usage and 3) we receive patient acknowledgement of assignment of benefits. We recognize rental revenue from electronic infusion pumps as earned, normally on a month-to-month basis. Pump rentals are billed at our established rates, which often differ from contractually allowable rates provided by third-party payors such as Medicare, Medicaid and commercial insurance carriers. All billings to third party payors are recorded net of provision for contractual adjustments to arrive at net revenues.
Due to the nature of the industry and the reimbursement environment in which we operate, certain estimates are required to record net revenues and accounts receivable at their net realizable values. Inherent in these estimates is the risk that they will have to be revised or updated as additional information becomes available. Specifically, the complexity of many third-party billing arrangements and the uncertainty of reimbursement amounts for certain services from certain payors may result in adjustments to amounts originally recorded. Due to continuing changes in the health care industry and third-party reimbursement, it is possible that management’s estimates could change in the near term, which could have an impact on our results of operations and cash flows.
27
Our largest payor is Medicare, which accounted for approximately 31% of our gross billings for the year ended December 31, 2010. We have contracts with various individual Blue Cross/Blue Shield affiliates which in the aggregate accounted for approximately 23% of our gross billings for the year ended December 31, 2010. No individual payor, other than Medicare and the Blue Cross/Blue Shield entities accounts for greater than 6% of our gross billings.
We recognize revenue for selling, renting and servicing new and pre-owned infusion pumps and other medical equipment to oncology practices as well as other alternate site settings including home care and home infusion providers, skilled nursing facilities, pain centers and others, when 1) persuasive evidence of an arrangement exists; 2) services have been rendered; 3) the price to the customer is fixed or determinable; and 4) collectability is reasonably assured. We perform an analysis to estimate sales returns and record an allowance. This estimate is based on historical sales returns.
Accounts Receivable and Allowance for Doubtful Accounts
Accounts receivable are reported at the estimated net realizable amounts from patients, third-party payors and other direct pay customers for goods provided and services rendered. We perform periodic analyses to assess the accounts receivable balances and record an allowance for doubtful accounts based on the estimated collectability of the accounts such that the recorded amounts reflect estimated net realizable value. Upon determination that an account is uncollectible, the account is written-off and charged to the allowance.
Accounts receivable are reduced by an allowance for amounts that could become uncollectible in the future. Our estimate for allowance for doubtful accounts is based upon management’s assessment of historical and expected net collections by payor. Due to continuing changes in the health care industry and third-party reimbursement it is possible that management’s estimates could change in the near term, which could have an impact on its financial position, results of operations, and cash flows.
Following is an analysis of the allowance for doubtful accounts for InfuSystem Holdings, Inc. for the years ended December 31, 2010, 2009 and 2008 ($000’s):
| Balance at beginning of Period |
Acquired in acquisition |
Charged to costs and expenses |
Deductions (1) | Balance at end of Period |
||||||||||||||||
| Allowance for doubtful accounts — 2010 |
$ | 1,842 | $ | 37 | $ | 4,515 | $ | (4,598 | ) | $ | 1,796 | |||||||||
| Allowance for doubtful accounts — 2009 |
$ | 1,552 | — | $ | 4,006 | $ | (3,716 | ) | $ | 1,842 | ||||||||||
| Allowance for doubtful accounts — 2008 |
$ | 1,638 | — | $ | 3,187 | $ | (3,273 | ) | $ | 1,552 | ||||||||||
| (1) | Deductions represent the write-off of uncollectible account receivable balances. |
Warrants and Derivative Financial Instruments
On April 18, 2006, we consummated our initial public offering (IPO) of 16,666,667 units. Each unit consists of one share of common stock and two redeemable common stock purchase warrants. Each warrant entitles the holder to purchase from us one share of our common stock at an exercise price of $5.00. On May 18, 2006, we sold an additional 208,584 units (the “Overallotment Units”) to FTN Midwest Securities Corp., the underwriter of our IPO (FTN Midwest), pursuant to a partial exercise by FTN Midwest of its overallotment option. The Warrant Agreement provides for us to register the shares underlying the warrants in the absence of our ability to deliver registered shares to the warrant holders upon warrant exercise.
ASC 815 requires freestanding derivative contracts that are settled in a company’s own stock, including common stock warrants, to be designated as equity instruments, assets or liabilities. Under the provisions of this standard, a contract designated as an asset or a liability must be carried at its fair value on a company’s balance sheet, with any changes in fair value recorded in the company’s results of operations. A contract designated as an equity instrument must be included within equity, and no fair value adjustments are required from period to period.
28
On February 16, 2010 we announced an Offer to Exchange common stock for outstanding warrants. At the time, we had 35,108,219 outstanding warrants. The exchange offer expired on March 17, 2010. Holders of our warrants had the option to exchange their warrants for either One (1) share of Common Stock for every thirty-five (35) Warrants tendered, or One (1) share of Common Stock for every twenty-five (25) Warrants tendered, provided the recipient agreed to be subject to a lock-up provision precluding transfer of the shares of Common Stock received for six months following the expiration of the Exchange Offer. The lock-up provision expired in September 2010. Based on the final count, 25,635,723 Warrants were properly tendered; 24,766,700 were tendered for shares of Common Stock subject to a lock-up, and 869,023 were tendered for unrestricted shares of Common Stock. Under the terms of the Exchange Offer, we issued an aggregate 1,015,489 shares of Common Stock in exchange for the tendered Warrants. After the exchange, there are 8,329,638 publicly held warrants and 1,142,858 privately held warrants outstanding.
In accordance with ASC 815, the 8,329,638 remaining warrants issued in connection with the IPO and overallotment to purchase common stock must be settled in registered shares and are separately accounted for as liabilities as discussed in Note 6. The fair value of these warrants is shown on our balance sheet and the unrealized changes in the value of these warrants are shown in our statement of operations as “Gain (loss) on derivatives.” These warrants are freely traded on the “Over the Counter Bulletin Board.” Consequently, the fair value of these warrants is estimated as the market price of the warrant at each period end. To the extent the market price increases or decreases, our warrant liabilities will also increase or decrease with a corresponding impact on the Company’s results of operations within “Gain (loss) on derivatives”.
Sales of warrants that can be settled in unregistered shares of common stock, as discussed in Note 10, are treated as equity and included in additional paid in capital. The total warrants issued to date that can be settled in unregistered shares of common stock are 1,142,858 at an issue price of $.70 per warrant or a total issue price of $800 thousand.
ASC 815 requires that an entity recognize all derivatives as either assets or liabilities in the statement of financial position and measure those instruments at fair value.
Cash Flow Hedge
We are exposed to risks associated with future cash flows related to the variability of the interest rate on its term loan with Bank of America. In order to manage the exposure of these risks, we enter into interest rate swaps. On July 20, 2010, we entered into a single interest rate swap and designated the swap as a cash flow hedge. In accordance with ASC 815, the fair value of the swap is shown on our consolidated balance sheet within derivative liabilities, unrealized changes in the fair value are included in accumulated other comprehensive loss within the stockholders’ equity section on our consolidated balance sheet, and any realized changes would be included in our consolidated statement of operations within interest expense.
Income Taxes
We account for income taxes in accordance with ASC 740, “Income Taxes,” which requires that we recognize deferred tax liabilities and assets based on the differences between the financial statement carrying amounts and the tax basis of assets and liabilities, using enacted tax rates in effect in the years the differences are expected to reverse. Deferred income tax (expense) benefit results from the change in net deferred tax assets or deferred tax liabilities. A valuation allowance is recorded when, in the opinion of management, it is more likely than not that some or all of any deferred tax assets will not be realized. For more information, refer to the “Income Taxes” discussion included in Note 8.
Goodwill Valuation
Goodwill arising from business combinations represents the excess of the purchase price over the estimated fair value of the net assets of the businesses acquired.
29
In accordance with the provisions of ASC 350, “Intangibles – Goodwill and Other,” goodwill is tested annually for impairment or more frequently if circumstances indicate the possibility of impairment. Significant judgments required to estimate fair value include estimating future cash flows, and determining appropriate discount rates, growth rates and other assumptions. Changes in these estimates and assumptions could materially affect the determination of fair value which could trigger impairment. We performed the annual impairment test at October 31, 2010, and determined there was no impairment of goodwill. The fair value of the Company’s single reporting unit was estimated using a valuation model that combined an income and market approach, utilizing the discounted cash flow and guideline public company methods, respectively, which indicated that the fair value of its net assets exceeded the carrying value by less than 10%. No events have occurred subsequent to October 31, 2010 that indicates impairment may have occurred.
The relationship of the Company’s market capitalization to the carrying value of its net assets can impact estimates of these assumptions, and can therefore impact the Company’s judgment as to the fair value of its reporting unit when performing goodwill impairment tests. During 2010, the Company’s market capitalization remained fairly consistent with such experience in 2009. The Company evaluated the movement in its stock price along with its 2010 performance relative to expectations. In addition, the Company assessed several unique factors; a thinly traded, closely held, illiquid stock, an “overhang” created by having a significant amount of warrants outstanding (see Note 6), and a limited research or analyst coverage. The implied control premium was within the range of market control premiums paid in transactions of companies in the healthcare industry during the past three years. Based on this evaluation, the Company concluded that neither the market capitalization at October 31, 2010, nor the change vs. the prior year, were definitive indicators of impairment.
As the Company’s warrants expire in April 2011 and its common stock was listed on the NY AMEX in December 2010, the Company will monitor the impact of these factors as well as control premiums for healthcare transactions, operational performance measures, general economic conditions and its market capitalization. A downward trend in one or more of these factors could cause the Company to reduce the estimated fair value of its reporting unit and recognize a corresponding impairment of goodwill in connection with a future goodwill impairment test.
| Item 7A. | Quantitative and Qualitative Disclosure About Market Risk. |
At December, 2010, the principal plus accrued interest on our term loan with Bank of America was $28.1 million. The term loan bears interest at LIBOR plus 4.5% or the Bank of America prime rate plus 3.5%, at our option. The loan is a variable rate loan and therefore fair value approximates book value. See Note 7 to our Consolidated Financial Statements included in this Annual Report on Form 10-K for further discussion of our term loan with Bank of America.
We are exposed to interest rate fluctuations on our underlying variable rate long-term debt. We utilize a single interest rate swap agreement to moderate approximately 65% of such exposure. We do not use derivative financial instruments for trading or other speculative purposes.
Based on the term loans and interest rate swaps outstanding, a decrease in LIBOR to zero (which is less than a 100 basis point decrease) and a 100 basis point decrease in the Bank of America prime rate would have increased our cash flow and pretax earnings by approximately $52 thousand for the year ended December 31, 2010. A 100 basis point increase in LIBOR and the Bank of America prime rate would have decreased our cash flow and pretax earnings year ended December 31, 2010 by approximately $14 thousand.
We have classified certain warrants as derivative liabilities, which resulted in a liability of $83 thousand at December 31, 2010. We classified the warrants as derivative liabilities because there is a possibility that we may be required to settle the warrants in registered shares of common stock. We are required to compare the fair market value of these instruments from the date of the initial recording to their fair market value as of the end of each reporting period and to reflect the change in fair market value in our Consolidated Statements of Operations as a gain or loss for the applicable period.
30
| Item 8. | Financial Statements and Supplementary Data. |
| Page | ||||
| 32 | ||||
| Consolidated Balance Sheets for the years ended December 31, 2010 and December 31, 2009 |
33 | |||
| 34 | ||||
| 35 | ||||
| 36 | ||||
| 38 | ||||
31

|
|
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and Stockholders of
InfuSystem Holdings, Inc.
Madison Heights, Michigan
We have audited the accompanying consolidated balance sheets of InfuSystem Holdings, Inc. and subsidiaries (the “Company”) as of December 31, 2010 and 2009, and the related consolidated statements of operations, stockholders’ equity, and cash flows for each of the three years in the period ended December 31, 2010. These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. Our audits included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion. An audit also includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, such consolidated financial statements present fairly, in all material respects, the financial position of InfuSystem Holdings, Inc. and subsidiaries as of December 31, 2010 and 2009, and the results of their operations and their cash flows for each of the three years in the period ended December 31, 2010, in conformity with accounting principles generally accepted in the United States of America.
/S/ DELOITTE & TOUCHE LLP
Detroit, Michigan
March 10, 2011

32
INFUSYSTEM HOLDINGS, INC. AND SUBSIDIARIES
CONSOLIDATED BALANCE SHEETS
| (in thousands, except share data) |
December 31, 2010 |
December 31, 2009 |
||||||
| ASSETS |
||||||||
| Current Assets: |
||||||||
| Cash and cash equivalents |
$ | 5,014 | $ | 7,750 | ||||
| Accounts receivable, less allowance for doubtful accounts of $1,796 and $1,842 at December 31, 2010 and 2009, respectively |
6,679 | 5,517 | ||||||
| Inventory |
1,699 | 925 | ||||||
| Prepaid expenses and other current assets |
750 | 395 | ||||||
| Deferred income taxes |
1,147 | 125 | ||||||
| Total Current Assets |
15,289 | 14,712 | ||||||
| Property & equipment, net |
16,672 | 13,499 | ||||||
| Deferred debt issuance costs, net |
658 | 781 | ||||||
| Goodwill |
64,092 | 56,580 | ||||||
| Intangible assets, net |
33,252 | 28,911 | ||||||
| Other assets |
401 | 207 | ||||||
| Total Assets |
$ | 130,364 | $ | 114,690 | ||||
| LIABILITIES AND STOCKHOLDERS’ EQUITY |
||||||||
| Current Liabilities: |
||||||||
| Accounts payable |
$ | 2,016 | $ | 1,306 | ||||
| Accrued expenses and other current liabilities |
4,631 | 1,573 | ||||||
| Derivative liabilities |
183 | 2,670 | ||||||
| Current portion of long-term debt |
5,551 | 5,501 | ||||||
| Total Current Liabilities |
12,381 | 11,050 | ||||||
| Long-term debt, net of current portion |
26,646 | 18,640 | ||||||
| Deferred income taxes |
5,788 | 3,314 | ||||||
| Other liabilities |
406 | 221 | ||||||
| Total Liabilities |
$ | 45,221 | $ | 33,225 | ||||
| Commitments and Contingencies |
— | — | ||||||
| Stockholders’ Equity |
||||||||
| Preferred stock, $.0001 par value: authorized 1,000,000 shares; none issued |
— | — | ||||||
| Common stock, $.0001 par value; authorized 200,000,000 shares; issued 21,163,337 and 18,734,144, respectively; outstanding 21,117,516 and 18,734,144, respectively |
2 | 2 | ||||||
| Additional paid-in capital |
87,004 | 81,410 | ||||||
| Accumulated other comprehensive loss |
(64 | ) | — | |||||
| Retained (deficit) earnings |
(1,799 | ) | 53 | |||||
| Total Stockholders’ Equity |
85,143 | 81,465 | ||||||
| Total Liabilities and Stockholders’ Equity |
$ | 130,364 | $ | 114,690 | ||||
See accompanying notes to consolidated financial statements.
33
INFUSYSTEM HOLDINGS, INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF OPERATIONS
| (in thousands, except share data) |
Year Ended December 31, 2010 |
Year Ended December 31, 2009 |
Year Ended December 31, 2008 |
|||||||||
| Net revenues |
$ | 47,229 | $ | 38,964 | $ | 35,415 | ||||||
| Cost of revenues: |
||||||||||||
| Product, service and supply costs |
7,730 | 6,200 | 5,422 | |||||||||
| Pump depreciation, sales and disposals |
5,954 | 4,127 | 3,769 | |||||||||
| Gross profit |
33,545 | 28,637 | 26,224 | |||||||||
| Sales, general and administrative expenses: |
||||||||||||
| Provision for doubtful accounts |
4,515 | 4,006 | 3,187 | |||||||||
| Amortization of intangibles |
2,259 | 1,827 | 1,827 | |||||||||
| Selling and marketing |
7,087 | 5,258 | 4,659 | |||||||||
| General administrative |
20,622 | 12,218 | 11,765 | |||||||||
| Total sales, general and administrative expenses |
34,483 | 23,309 | 21,438 | |||||||||
| Operating (loss) income |
(938 | ) | 5,328 | 4,786 | ||||||||
| Other (loss) income: |
||||||||||||
| Gain (loss) on derivatives |
207 | (78 | ) | 9,815 | ||||||||
| Interest expense |
(3,352 | ) | (3,499 | ) | (3,735 | ) | ||||||
| Gain on extinguishment of long-term debt |
1,118 | — | — | |||||||||
| Other expense |
(258 | ) | — | — | ||||||||
| Total other (loss) income |
(2,285 | ) | (3,577 | ) | 6,080 | |||||||
| (Loss) income before income taxes |
(3,223 | ) | 1,751 | 10,866 | ||||||||
| Income tax benefit (expense) |
1,371 | (977 | ) | (907 | ) | |||||||
| Net (loss) income |
$ | (1,852 | ) | $ | 774 | $ | 9,959 | |||||
| Net (loss) income per share: |
||||||||||||
| Basic |
$ | (0.09 | ) | $ | 0.04 | $ | 0.56 | |||||
| Diluted |
$ | (0.09 | ) | $ | 0.04 | $ | 0.53 | |||||
| Weighted average shares outstanding: |
||||||||||||
| Basic |
19,721,378 | 18,609,797 | 17,940,952 | |||||||||
| Diluted |
19,721,378 | 18,931,356 | 18,672,321 | |||||||||
See accompanying notes to consolidated financial statements.
34
INFUSYSTEM HOLDINGS, INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF
STOCKHOLDERS’ EQUITY
| Common Stock | Treasury Stock | Total Stockholders’ Equity |
||||||||||||||||||||||||||||||
| (in thousands, except share data) |
Shares | Par Value $0.0001 Amount |
Paid in Capital in Excess of Par |
Retained (Deficit) Earnings |
Accumulated Other Comprehensive Loss |
Shares | Amount | |||||||||||||||||||||||||
| Balances at January 1, 2008 |
18,316 | 2 | $ | 79,437 | $ | (10,680 | ) | $ | — | (1,491 | ) | $ | — | $ | 68,759 | |||||||||||||||||
| Gross restricted shares issued upon vesting |
275 | — | — | — | — | — | — | — | ||||||||||||||||||||||||
| Amortization of stock-based compensation expense |
— | — | 1,550 | — | — | — | — | 1,550 | ||||||||||||||||||||||||
| Issuance of treasury stock for services |
— | — | — | — | — | 257 | — | — | ||||||||||||||||||||||||
| Common stock repurchased to satisfy minimum statutory withholding on stock-based compensation |
(78 | ) | — | (195 | ) | — | — | — | — | (195 | ) | |||||||||||||||||||||
| Net income |
— | — | — | 9,959 | — | — | — | 9,959 | ||||||||||||||||||||||||
| Balances at December 31, 2008 |
18,513 | 2 | $ | 80,792 | $ | (721 | ) | $ | — | (1,234 | ) | $ | — | $ | 80,073 | |||||||||||||||||
| Gross restricted shares issued upon vesting |
265 | — | — | — | — | — | — | — | ||||||||||||||||||||||||
| Common stock issued to employees |
8 | |||||||||||||||||||||||||||||||
| Amortization of stock-based compensation expense |
— | — | 753 | — | — | — | — | 753 | ||||||||||||||||||||||||
| Issuance of treasury stock for services |
— | — | — | — | — | 1,234 | — | — | ||||||||||||||||||||||||
| Common stock repurchased to satisfy minimum statutory withholding on stock-based compensation |
(52 | ) | — | (135 | ) | — | — | — | — | (135 | ) | |||||||||||||||||||||
| Net income |
— | — | — | 774 | — | — | — | 774 | ||||||||||||||||||||||||
| Balances at December 31, 2009 |
18,734 | 2 | $ | 81,410 | $ | 53 | $ | — | — | $ | — | $ | 81,465 | |||||||||||||||||||
| Gross restricted shares issued upon vesting |
1,476 | — | — | — | — | — | — | — | ||||||||||||||||||||||||
| Common stock issued to employees |
5 | |||||||||||||||||||||||||||||||
| Shares issued from warrant exchange |
1,015 | 2,015 | 2,015 | |||||||||||||||||||||||||||||
| Amortization of stock-based compensation expense |
— | — | 3,860 | — | — | — | — | 3,860 | ||||||||||||||||||||||||
| Treasury shares repurchased |
— | — | (114 | ) | — | — | (46 | ) | — | (114 | ) | |||||||||||||||||||||
| Common stock repurchased to satisfy minimum statutory withholding on stock-based compensation |
(67 | ) | — | (167 | ) | — | — | — | — | (167 | ) | |||||||||||||||||||||
| Net loss |
— | — | — | (1,852 | ) | — | — | (1,852 | ) | |||||||||||||||||||||||
| Comprehensive loss |
(64 | ) | (64 | ) | ||||||||||||||||||||||||||||
| Balances at December 31, 2010 |
21,163 | 2 | $ | 87,004 | $ | (1,799 | ) | $ | (64 | ) | (46 | ) | $ | — | $ | 85,143 | ||||||||||||||||
See accompanying notes to consolidated financial statements.
35
INFUSYSTEM HOLDINGS, INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF CASH FLOWS
| (in thousands) |
Year Ended December 31, 2010 |
Year Ended December 31, 2009 |
Year Ended December 31, 2008 |
|||||||||
| OPERATING ACTIVITIES |
||||||||||||
| Net (loss) income |
$ | (1,852 | ) | $ | 774 | $ | 9,959 | |||||
| Adjustments to reconcile net (loss) income to net cash provided by operating activities: |
||||||||||||
| (Gain) loss on derivative liabilities |
(207 | ) | 78 | (9,815 | ) | |||||||
| (Gain) on extinguishment of long-term debt |
(1,118 | ) | — | — | ||||||||
| Provision for doubtful accounts |
4,515 | 4,006 | 3,187 | |||||||||
| Depreciation |
5,357 | 4,122 | 3,935 | |||||||||
| Loss on disposal of pumps |
994 | 342 | 553 | |||||||||
| Amortization of intangible assets |
2,259 | 1,827 | 1,827 | |||||||||
| Amortization of deferred debt issuance costs |
980 | 495 | 642 | |||||||||
| Stock-based compensation |
3,860 | 753 | 1,550 | |||||||||
| Deferred income taxes |
(1,236 | ) | 2,254 | 935 | ||||||||
| Changes in assets and liabilities, exclusive of effects of acquisitions: |
||||||||||||
| (Increase) in accounts receivable, net of provision |
(3,948 | ) | (5,355 | ) | (1,835 | ) | ||||||
| (Increase) decrease in other current assets |
(506 | ) | (253 | ) | 560 | |||||||
| (Increase) in other assets |
(173 | ) | (207 | ) | — | |||||||
| Increase (decrease) in accounts payable and other liabilities |
2,252 | 872 | (601 | ) | ||||||||
| (Decrease) in derivative liabilities from termination of interest rate swap |
(365 | ) | — | — | ||||||||
| NET CASH PROVIDED BY OPERATING ACTIVITIES |
10,812 | 9,708 | 10,897 | |||||||||
| INVESTING ACTIVITIES |
||||||||||||
| Capital expenditures |
(2,444 | ) | (4,612 | ) | (1,733 | ) | ||||||
| Cash paid for acquisition, net of cash acquired |
(16,616 | ) | — | — | ||||||||
| Proceeds from sale of property |
— | 1 | 10 | |||||||||
| Payment of deferred acquisition costs |
— | — | (105 | ) | ||||||||
| Cash received for acquisition from I-Flow |
— | — | 784 | |||||||||
| NET CASH USED IN INVESTING ACTIVITIES |
(19,060 | ) | (4,611 | ) | (1,044 | ) | ||||||
| FINANCING ACTIVITIES |
||||||||||||
| Principal payments on term loan |
(22,623 | ) | (8,565 | ) | (2,044 | ) | ||||||
| Cash proceeds from term loan |
30,000 | — | — | |||||||||
| Capitalized debt issuance costs |
(808 | ) | — | — | ||||||||
| Common stock repurchased to satisfy minimum statutory withholding on stock-based compensation |
(167 | ) | (135 | ) | (195 | ) | ||||||
| Treasury shares repurchased |
(68 | ) | — | — | ||||||||
| Principal payments on capital lease obligations |
(822 | ) | (160 | ) | (61 | ) | ||||||
| NET CASH PROVIDED BY (USED IN) FINANCING ACTIVITIES |
5,512 | (8,860 | ) | (2,300 | ) | |||||||
| Net change in cash and cash equivalents |
(2,736 | ) | (3,763 | ) | 7,553 | |||||||
| Cash and cash equivalents, beginning of period |
7,750 | 11,513 | 3,960 | |||||||||
| Cash and cash equivalents, end of period |
$ | 5,014 | $ | 7,750 | $ | 11,513 | ||||||
36
The following table presents certain supplementary cash flow information for the years ended December 31, 2010, 2009 and 2008:
| (in thousands) |
Year Ended December 31, 2010 |
Year Ended December 31, 2009 |
Year Ended December 31, 2008 |
|||||||||
| Cash paid for interest (including swap payments) |
$ | 2,372 | $ | 2,933 | $ | 3,115 | ||||||
| Cash paid for income taxes |
$ | 21 | $ | 18 | $ | 533 | ||||||
| Supplementary non-cash activities: |
||||||||||||
| Property acquired with a capital lease |
$ | 1,869 | $ | 2,198 | $ | 480 | ||||||
| Tender offer to exchange warrants |
$ | 2,016 | $ | — | $ | — | ||||||
| Additions to property (a) |
$ | 903 | $ | 291 | $ | 14 | ||||||
| Origination of seller note (b) |
$ | 750 | $ | — | $ | — | ||||||
| Current assets assumed in acquisition (b) |
$ | 2,352 | $ | — | $ | — | ||||||
| Current liabilities assumed in acquisition (b) |
$ | 438 | $ | — | $ | — | ||||||
| Deferred tax liability assumed in acquisition (b) |
$ | 2,754 | $ | — | $ | — | ||||||
| Deferred tax asset assumed in acquisition (b) |
$ | 30 | $ | — | $ | — | ||||||
| Treasury stock transactions (number of shares) |
46 | 1,234 | 257 | |||||||||
| Gross issuance of vested restricted shares (number of shares) |
1,476 | 265 | 275 | |||||||||
| (a) | Amounts consist of current liabilities for net property that have not been included in investing activities. These amounts have not been paid for as of December 31, 2010, 2009 and 2008 but will be included as a cash outflow when paid. |
| (b) | See Note 3 — “Acquisitions” |
See accompanying notes to consolidated financial statements.
37
INFUSYSTEM HOLDINGS, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
| 1. | Basis of Presentation and Nature of Operations |
The information in this Annual Report on Form 10-K includes the financial position of InfuSystem Holdings, Inc. and its consolidated subsidiaries (the “Company”) as of December 31, 2010 and 2009, the results of its operations and cash flows for the years ended December 31, 2010, 2009 and 2008, and stockholders’ equity from January 1, 2008 to December 31, 2010. In the opinion of the Company, the consolidated statements for the all periods presented include all adjustments, consisting of normal recurring adjustments, necessary to present a fair statement of the results for such periods.
The accompanying consolidated financial statements have been prepared in accordance with accounting principles generally accepted in the United States of America (“GAAP”). All intercompany accounts and transactions have been eliminated.
The Company is the leading provider of infusion pumps and related services. The Company services hospitals, oncology practices and other alternate site healthcare providers. Headquartered in Madison Heights, Michigan, the Company delivers local, field-based customer support, and also operates pump repair Centers of Excellence in Michigan, Kansas, California, and Ontario, Canada.
On June 15, 2010, the Company entered into a stock purchase agreement with the shareholders of First Biomedical, Inc., (First Biomedical) a Kansas corporation, to acquire all of the issued and outstanding stock of First Biomedical and completed the acquisition simultaneously. First Biomedical sells, rents, services and repairs new and pre-owned infusion pumps and other medical equipment. First Biomedical also sells a variety of primary and secondary tubing, cassettes, catheters and other disposable items that are utilized with infusion pumps. For more information, refer to the “Acquisition” discussion included in Note 3.
The Company supplies electronic ambulatory infusion pumps and associated disposable supply kits to oncology practices, infusion clinics and hospital outpatient chemotherapy clinics. These pumps and supplies are utilized primarily by colorectal cancer patients who receive a standard of care treatment that utilizes continuous chemotherapy infusions delivered via electronic ambulatory infusion pumps. The Company obtains an assignment of insurance benefits from the patient, bills the insurance company or patient accordingly, and collects payment. The Company provides pump management services for the pumps and associated disposable supply kits to over 1,300 oncology practices in the United States. The Company retains title to the pumps during this process.
In addition, the Company sells or rents new and pre-owned pole mounted and ambulatory infusion pumps to, and provides biomedical recertification, maintenance and repair services for oncology practices as well as other alternate site settings including home care and home infusion providers, skilled nursing facilities, pain centers and others. The Company also provides these products and services to customers in the small-hospital market.
The Company purchases new and pre-owned pole mounted and ambulatory infusion pumps from a variety of sources on a non-exclusive basis. The Company repairs, refurbishes and provides biomedical certification for the devices as needed. The pumps are then available for sale, rental or to be used within the Company’s ambulatory infusion pump management service.
| 2. | Summary of Significant Accounting Policies |
Principles of Consolidation
The consolidated financial statements include the accounts of the Company and all wholly owned organizations. All intercompany transactions and account balances have been eliminated in consolidation.
38
Segments
The Company operates in one business segment based on management’s view of its business for purposes of evaluating performance and making operating decisions, representing the only reportable segment in accordance with Accounting Standard Codification (“ASC”) 280, “Segment Reporting.”
The Company utilizes shared services including but not limited to, human resources, payroll, finance, sales, pump repair and maintenance services, as well as certain shared assets and sales, general and administrative costs. The Company is in the process of transitioning more shared services and synergies since the acquisition of First Biomedical. The Company’s approach is to make operational decisions and assess performance based on delivering products and services that together provide solutions to our customer base, utilizing functional management structure and shared services where possible. Based upon this business model, the chief operating decision maker only reviews consolidated financial information.
Use of Estimates
The preparation of financial statements in conformity with GAAP requires management to make estimates, assumptions and judgments that affect the amounts reported in the financial statements, including the notes thereto. The Company considers critical accounting policies to be those that require more significant judgments and estimates in the preparation of its consolidated financial statements, including the following: revenue recognition, which includes contractual adjustments; accounts receivable and allowance for doubtful accounts; sales return allowances; inventory reserves; income taxes; and goodwill valuation. Management relies on historical experience and other assumptions believed to be reasonable in making its judgment and estimates. Actual results could differ materially from those estimates.
Cash and Cash Equivalents
The Company considers all highly liquid investments with original maturities of three months or less to be cash equivalents. The Company maintains its cash and cash equivalents primarily with two financial institutions and is fully insured with the Federal Deposit Insurance Corporation (FDIC) under the Temporary Liquidity Guarantee Program until December 31, 2012.
Accounts Receivable and Allowances
Accounts receivable are reported at the estimated net realizable amounts from patients, third-party payors and other direct pay customers for goods provided and services rendered. The Company performs periodic analyses to assess the accounts receivable balances. It records an allowance for doubtful accounts based on the estimated collectability of the accounts such that the recorded amounts reflect estimated net realizable value. Upon determination that an account is uncollectible, the account is written-off and charged to the allowance.
Accounts receivable are reduced by an allowance for amounts that could become uncollectible in the future. The Company’s estimate for its allowance for doubtful accounts is based upon management’s assessment of historical and expected net collections by payor. Due to continuing changes in the health care industry and third-party reimbursement it is possible that management’s estimates could change in the near term, which could have an impact on its financial position, results of operations, and cash flows.
Following is an analysis of the allowance for doubtful accounts for InfuSystem Holdings, Inc. for the years ended December 31, 2010, 2009 and 2008 ($000’s):
| Balance at beginning of Period |
Acquired in acquisition |
Charged to costs and expenses |
Deductions (1) | Balance at end of Period |
||||||||||||||||
| Allowance for doubtful accounts — 2010 |
$ | 1,842 | $ | 37 | $ | 4,515 | $ | (4,598 | ) | $ | 1,796 | |||||||||
| Allowance for doubtful accounts — 2009 |
$ | 1,552 | — | $ | 4,006 | $ | (3,716 | ) | $ | 1,842 | ||||||||||
| Allowance for doubtful accounts — 2008 |
$ | 1,638 | — | $ | 3,187 | $ | (3,273 | ) | $ | 1,552 | ||||||||||
| (1) | Deductions represent the write-off of uncollectible account receivable balances. |
39
Inventory
Our Inventory consists of infusion pumps and related parts and supplies and is stated at the lower of cost, determined on a first in, first out basis, or market. The Company periodically performs an analysis of slow moving inventory and records a reserve based on estimated obsolete inventory.
Property and Equipment
Property and equipment is stated at acquired cost and depreciated using the straight-line method over the estimated useful lives of the related assets, ranging from three to seven years. Rental equipment, consisting primarily of infusion pumps that the Company acquires from third-parties, is depreciated over five years. Information Technology (IT) software and hardware are depreciated over three years. Leasehold improvements are amortized using the straight-line method over the life of the asset or the remaining term of the lease, whichever is shorter. Maintenance and minor repairs are charged to operations as incurred. When assets are sold, or otherwise disposed of, the cost and related accumulated depreciation are removed from the accounts and any gain or loss is recorded in the current period.
Long-Lived Assets
The Company accounts for the impairment and disposition of long-lived assets in accordance with ASC 360, “Property, Plant and Equipment.” This standard addresses financial accounting and reporting for the impairment of long-lived assets and for the disposal of long-lived assets. In accordance with this standard, long-lived assets to be held are reviewed for events or changes in circumstances, which indicate that their carrying value may not be recoverable. If an impairment indicator exists, the Company assesses the asset or asset group for recoverability. Recoverability of these assets is determined based upon the expected undiscounted future net cash flows from the operations to which the assets relate, utilizing management’s best estimates, appropriate assumptions and projections at the time. If the carrying value is determined not to be recoverable from future operating cash flows, the asset is deemed impaired and an impairment loss would be recognized to the extent the carrying value exceeded the estimated fair market value of the asset. The Company reviews the carrying value of long-lived assets if there is an indicator of impairment. The Company has determined that no impairment indicators existed as of December 31, 2010.
Goodwill Valuation
Goodwill arising from business combinations represents the excess of the purchase price over the estimated fair value of the net assets of the businesses acquired.
In accordance with the provisions of ASC 350, “Intangibles — Goodwill and Other,” goodwill is tested annually for impairment or more frequently if circumstances indicate the possibility of impairment. Significant judgments required to estimate fair value include estimating future cash flows, and determining appropriate discount rates, growth rates and other assumptions. Changes in these estimates and assumptions could materially affect the determination of fair value which could trigger impairment. The Company performed the annual impairment test at October 31, 2010, and determined there was no impairment of goodwill. No events have occurred subsequent to October 31, 2010 that indicates impairment may have occurred. For more information, refer to the “Goodwill and Intangible Assets” discussion included in Note 5.
Intangible Assets
Intangible assets consist of trade names, physician and customer relationships, non-compete agreements, and software. The trade names, physician and customer relationships and non-compete agreements arose from the acquisitions of InfuSystem and First Biomedical. The Company amortizes the value assigned to the physician and customer relationships on a straight-line basis over the period of expected benefit, which is 15 years. The
40
acquired physician and customer relationship base represents a valuable asset of InfuSystem due to the expectation of future business opportunities to be leveraged from the existing relationship with each physician and customer. InfuSystem has long-standing relationships with numerous oncology clinics, physicians, home care and home infusion providers, skilled nursing facilities, pain centers and others. These relationships are expected, on average, to have a 15 year useful life, based on minimal attrition experienced to date by the Company and expectations of continued minimal attrition. Non-compete agreements are amortized on a straight-line basis over five years and software is amortized on a straight-line basis over three years. Management tests non-amortizable intangible assets (i.e., trade names such as InfuSystem) for impairment in accordance with ASC 350. The Company performed the annual impairment test at October 31, 2010, and determined there was no impairment. No events have occurred subsequent to October 31, 2010 that indicates impairment may have occurred. For more information, refer to the “Goodwill and Intangible Assets” discussion included in Note 5.
Accumulated Other Comprehensive Income (Loss)
Accumulated other comprehensive loss consists only of the unrealized loss on the single interest rate swap in place as of December 31, 2010, net of taxes. For more information on the interest rate swap, refer to Note 6. During the year there was an accumulated other comprehensive loss of $100 thousand related to the unrealized loss on the swap. The tax impact on the loss was $35 thousand, leaving a net accumulated other comprehensive loss of $64 thousand. These were the only net changes to accumulated other comprehensive loss for the year ended December 31, 2010. The following table summarizes comprehensive loss for the applicable periods (in thousands):
| Year
Ended December 31, 2010 |
||||
| Net income (loss) |
$ | (1,852 | ) | |
| Accumulated other comprehensive income (loss) on derivatives, net of taxes |
(64 | ) | ||
| Total comprehensive income (loss) |
$ | (1,916 | ) | |
Revenue Recognition
The majority of the Company’s revenue is rental revenue in the oncology market. Revenues are recognized predominantly under fee for service arrangements through equipment that the Company rents to patients. The Company recognizes revenue only when all of the following criteria are met: 1) persuasive evidence of an arrangement exists; 2) services have been rendered; 3) the price to the customer is fixed or determinable; and 4) collectability is reasonably assured. Persuasive evidence of an arrangement is determined to exist, and collectability is reasonably assured, when the Company receives 1) a physician’s written order and assignment of benefits, signed by the physician and patient, respectively, and the Company has 2) verified actual pump usage and 3) insurance coverage. The Company recognizes rental revenue from electronic infusion pumps as earned, normally on a month-to-month basis. Pump rentals are billed at the Company’s established rates, which often differ from contractually allowable rates provided by third-party payors such as Medicare, Medicaid and commercial insurance carriers. All billings to third party payors are recorded net of provision for contractual adjustments to arrive at net revenues.
Due to the nature of the industry and the reimbursement environment in which the Company operates, certain estimates are required to record net revenues and accounts receivable at their net realizable values. Inherent in these estimates is the risk that they will have to be revised or updated as additional information becomes available. Specifically, the complexity of many third-party billing arrangements and the uncertainty of reimbursement amounts for certain services from certain payors may result in adjustments to amounts originally recorded. Due to continuing changes in the health care industry and third-party reimbursement, it is possible that management’s estimates could change in the near term, which could have an impact on our results of operations and cash flows.
41
The Company’s largest payor is Medicare, which accounted for approximately 31%, 31% and 32% of its gross billings for ambulatory infusion pump services for the years ended December 31, 2010, 2009 and 2008, respectively. The Company has contracts with various individual Blue Cross/Blue Shield affiliates which in the aggregate accounted for approximately 23%, 22% and 22% of its gross billings for ambulatory infusion pump services for the years ended December 31, 2010, 2009 and 2008, respectively. No individual payor (other than Medicare and the Blue Cross/Blue Shield entities) accounts for greater than 6% of the Company’s ambulatory infusion pump services gross billings for the fiscal years ended December 31, 2010, 2009 and 2008.
The Company recognizes revenue for selling, renting and servicing new and pre-owned infusion pumps and other medical equipment to oncology practices as well as other alternate site settings including home care and home infusion providers, skilled nursing facilities, pain centers and others, when persuasive evidence of an arrangement exists; services have been rendered; the price to the customer is fixed or determinable; and collectability is reasonably assured. The Company performs an analysis to estimate sales returns and records an allowance. This estimate is based on historical sales returns.
Income Taxes
The Company accounts for income taxes in accordance with ASC 740, “Income Taxes,” which requires that the Company recognize deferred tax liabilities and assets based on the differences between the financial statement carrying amounts and the tax basis of assets and liabilities, using enacted tax rates in effect in the years the differences are expected to reverse. Deferred income tax (expense) benefit results from the change in net deferred tax assets or deferred tax liabilities. A valuation allowance is recorded when, in the opinion of management, it is more likely than not that some or all of any deferred tax assets will not be realized. For more information, refer to the “Income Taxes” discussion included in Note 8.
Share Based Payment
ASC 718, “Stock Compensation,” requires all entities to recognize compensation expense in an amount equal to the fair value of share based payments made to employees, among other requirements. Under the fair value based method, compensation cost is measured at the grant date based on the fair value of the award and is recognized on a straight-line basis over the award vesting period. Accordingly, share based payments issued to officers and directors are measured at fair value and recognized as expense over the related vesting periods.
In 2007, the Company adopted the 2007 Stock Incentive Plan (the “Plan”) providing for the issuance of a maximum of 2,000,000 shares of common stock in connection with the grant of stock-based or stock-denominated awards. In addition, during the year ended December 31, 2010, the Company made certain grants of restricted stock outside of the Plan.
During the year ended December 31, 2010, the Company granted 3,440,000 restricted shares. Of the total shares granted, 1,440,000 entitle a holder to receive, at the end of a vesting period, a specified number of shares of the Company’s common stock. The remaining 2,000,000 shares granted entitle the holder to receive common stock when the shares vest based upon certain market conditions tied to the Company’s stock price, or certain performance conditions including a change in control.
Share based compensation expense recognized for the year ended December 31, 2010, 2009 and 2008 was $5.9 million, $753 thousand and $1.6 million, respectively.
Warrants and Derivative Financial Instruments
On April 18, 2006, the Company consummated its initial public offering (“IPO”) of 16,666,667 units. Each unit consists of one share of common stock and two redeemable common stock purchase warrants. Each warrant entitles the holder to purchase from the Company one share of its common stock at an exercise price of $5.00. On May 18, 2006, the Company sold an additional 208,584 units (the “Overallotment Units”) to FTN Midwest Securities Corp., the underwriter of its IPO (FTN Midwest), pursuant to a partial exercise by FTN Midwest of its
42
overallotment option. The Warrant Agreement provides for the Company to register the shares underlying the warrants in the absence of the Company’s ability to deliver registered shares to the warrant holders upon warrant exercise.
ASC 815 requires freestanding derivative contracts that are settled in a company’s own stock, including common stock warrants, to be designated as equity instruments, assets or liabilities. Under the provisions of this standard, a contract designated as an asset or a liability must be carried at its fair value on a company’s balance sheet, with any changes in fair value recorded in the company’s results of operations. A contract designated as an equity instrument must be included within equity, and no fair value adjustments are required from period to period.
On February 16, 2010 the Company announced an Offer to Exchange common stock for outstanding warrants. At the time, the Company had 35,108,219 outstanding warrants. The exchange offer expired on March 17, 2010. Holders of the Company’s warrants had the option to exchange their warrants for either One (1) share of Common Stock for every thirty-five (35) Warrants tendered, or One (1) share of Common Stock for every twenty-five (25) Warrants tendered, provided the recipient agreed to be subject to a lock-up provision precluding transfer of the shares of Common Stock received for six months following the expiration of the Exchange Offer. The lock-up provision expired in September 2010. Based on the final count, 25,635,723 Warrants were properly tendered; 24,766,700 were tendered for shares of Common Stock subject to a lock-up, and 869,023 were tendered for unrestricted shares of Common Stock. Under the terms of the Exchange Offer, the Company issued an aggregate 1,015,489 shares of Common Stock in exchange for the tendered Warrants. After the exchange, there are 8,329,638 publicly held warrants and 1,142,858 privately held warrants outstanding. The Company recognized a loss of $491 thousand as a result of the exchange.
In accordance with ASC 815, the 8,329,638 remaining warrants issued in connection with the IPO and overallotment to purchase common stock must be settled in registered shares and are separately accounted for as liabilities as discussed in Note 6. The fair value of these warrants is shown on the Company’s balance sheet and the unrealized changes in the value of these warrants are shown in the Company’s statement of operations as “Gain (loss) on derivatives.” These warrants are freely traded on the “Over the Counter Bulletin Board.” Consequently, the fair value of these warrants is estimated as the market price of the warrant at each period end. To the extent the market price increases or decreases, the Company’s warrant liabilities will also increase or decrease with a corresponding impact on the Company’s results of operations within “Gain (loss) on derivatives”.
Sales of warrants that can be settled in unregistered shares of common stock, as discussed in Note 10, are treated as equity and included in additional paid in capital. The total warrants issued to date that can be settled in unregistered shares of common stock are 1,142,858 at an issue price of $.70 per warrant or a total issue price of $800 thousand.
ASC 815 requires that an entity recognize all derivatives as either assets or liabilities in the statement of financial position and measure those instruments at fair value.
Cash Flow Hedge
The Company is exposed to risks associated with future cash flows related to the variability of the interest rate on its term loan with Bank of America. In order to manage the exposure of these risks, the Company enters into interest rate swaps. On July 20, 2010, the Company entered into a single interest rate swap and designated the swap as a cash flow hedge. In accordance with ASC 815, the fair value of the swap is shown on the Company’s consolidated balance sheet within derivative liabilities, unrealized changes in the fair value are included in accumulated other comprehensive loss within the stockholders’ equity section on the Company’s consolidated balance sheet, and any realized changes would be included in the Company’s consolidated statement of operations within interest expense.
43
Deferred Debt Issuance Costs
Capitalized debt issuance costs as of December 31, 2010 relate solely to the Company’s Bank of America credit facility, while as of December 31, 2009 they related solely to the Company’s term loan with Kimberly-Clark (formerly I-Flow). The Company classifies the costs related to the Bank of America credit facility as non-current assets and amortizes them using the interest method through the maturity date of June 2014. For a further discussion of the Company’s deferred debt issuance costs, see Note 7.
Earnings Per Share
Basic earnings (loss) per share is computed by dividing net income (loss) by the weighted average number of common shares outstanding during the period. Diluted earnings (loss) per share assumes the issuance of potentially dilutive shares of common stock during the periods. The following table reconciles the numerators and denominators of basic and diluted earnings (loss) per share computations:
| Year Ended December 31, 2010 |
Year Ended December 31, 2009 |
Year Ended December 31, 2008 |
||||||||||
| Numerator: |
||||||||||||
| Net (loss) income (in thousands) |
$ | (1,852 | ) | $ | 774 | $ | 9,959 | |||||
| Denominator: |
||||||||||||
| Weighted average common shares outstanding: |
||||||||||||
| Basic |
19,721,378 | 18,609,797 | 17,940,952 | * | ||||||||
| Dilutive effect of non-vested awards |
— | 321,559 | 731,369 | |||||||||
| Diluted |
19,721,378 | 18,931,356 | 18,672,321 | |||||||||
| Net (loss) earnings per share: |
||||||||||||
| Basic |
$ | (0.09 | ) | $ | 0.04 | $ | 0.56 | |||||
| Diluted |
$ | (0.09 | ) | $ | 0.04 | $ | 0.53 | |||||
| * | Includes, from April 25, 2008, the 1,234,044 shares referenced in Note 9 to our Consolidated Financial Statements included in this Annual Report on Form 10-K. As of December 31, 2008, the Company was in the process of taking necessary administrative steps to effectuate issuance of the remaining 1,234,044 shares which were issued in February 2009. |
For the year ended December 31, 2010, the following warrants, stock options and restricted shares were not included in the calculation because they would have an anti-dilutive effect because of the net loss: 8,329,638 outstanding warrants issued in connection with the IPO, 1,142,858 warrants issued privately, 130,479 vested stock options and 2,173,250 in unvested restricted shares. For the years ended December 31, 2009 and 2008, the following warrants were not included in the calculation because they would have an anti-dilutive effect: 33,750,502 outstanding warrants issued in connection with the IPO and 1,357,717 warrants issued privately. For the year ended December 31, 2009, there were 100,479 vested stock options granted under the 2007 Stock Incentive Plan that were not included in the calculation as they would have an anti-dilutive effect. For the year ended December 31, 2008, there were 300,000 non-vested stock options granted under the 2007 Stock Incentive Plan that were not included in the calculation as they would have an anti-dilutive effect.
Subsequent events
The Company adopted the provisions of ASC 855, “Subsequent Events” effective June 15, 2009, and management has concluded that there are no other significant subsequent events requiring disclosure as of the date the consolidated financial statements were issued.
44
| 3. | Acquisitions |
Entry into a Material Definitive Agreement
On June 15, 2010, the Company entered into a stock purchase agreement with the shareholders of First Biomedical to acquire all of the issued and outstanding stock of First Biomedical and completed the acquisition for total consideration of $17.4 million. Included in the consideration is $16.7 million paid in cash and a $750 thousand seller note described in further detail below.
First Biomedical sells, rents, services and repairs new and pre-owned infusion pumps and other medical equipment. First Biomedical also sells a variety of primary and secondary tubing, cassettes, catheters and other disposable items that are utilized with infusion pumps. Headquartered in Olathe, KS, with additional facilities in California and Toronto, First Biomedical is a leading provider to alternate site healthcare facilities and hospitals in the United States and Canada. The acquisition of First Biomedical allows the Company to expand its offerings to existing customers with the addition of biomedical service and repair, while simultaneously bolstering the growth of infusion pump sales within the oncology space and realized synergies.
First Biomedical’s results of operations are included in the Company’s consolidated statements of operations from the acquisition date.
Purchase Price Allocation
Pursuant to ASC 805, “Business Combinations,” the purchase price has been allocated to the assets acquired and liabilities assumed based upon their estimated fair values as of the acquisition date. The purchase price allocation was primarily based upon a valuation using income and cost approaches, and management’s estimates and assumptions. There was an excess, or premium, paid for the acquisition due to the benefits described above. The excess of the purchase price over the net tangible and identifiable intangible assets was recorded as goodwill. For tax purposes, goodwill consists of both identifiable intangible assets (customer relationships and non-competition agreements from the table below) and unidentifiable intangible assets (goodwill from the table below). Goodwill is expected to be partially deductible for tax purposes. The purchase price allocation is based on a final analysis. The allocation of the purchase price to the fair values of the assets acquired and liabilities assumed as of the transaction date is presented below (in thousands):
| Accounts receivable, net of allowances |
$ | 1,729 | ||
| Other current assets |
700 | |||
| Property and equipment |
4,772 | |||
| Goodwill |
7,512 | |||
| Customer relationships |
5,000 | |||
| Non-competition agreements |
760 | |||
| Other assets |
131 | |||
| Current liabilities |
(438 | ) | ||
| Deferred tax liability |
(2,754 | ) | ||
| Total purchase price |
$ | 17,412 | ||
The stock purchase agreement provided for an adjustment to the purchase price based on final working capital as of the closing balance sheet, which was finalized during the fourth quarter of year ended December 31, 2010 and resulted in an additional payment of $199 thousand, increasing the total purchase price.
Acquired property and equipment are being depreciated on a straight-line basis with estimated remaining lives ranging from 1 year to 14.5 years. Intangible assets are being amortized on a straight-line basis with estimated remaining lives ranging from 5 to 15 years reflecting the expected future value.
45
Fees
During the year ended December 31, 2010, we incurred legal and professional fees directly related to the First Biomedical acquisition totaling approximately $965 thousand. All such costs are presented under the caption “General and administrative” within operating expenses in the accompanying consolidated statements of operations.
Seller Note
Pursuant to the terms of the Stock Purchase Agreement, as of the date of the acquisition the Company entered into a subordinated promissory note with the former majority shareholder of First Biomedical (the Seller) in the amount of $750 thousand. In accordance with the note, the Company will pay the Seller in equal installments over 24 months, which includes annual interest of 5%. As of December 31, 2010 the outstanding principal due on the note was $569 thousand.
Pro Forma Financial Information
The pro forma financial information in the table below summarizes the combined results of operations of the Company and First Biomedical as though the companies had been combined as of the beginning of each period presented. The pro forma financial information is presented for informational purposes only and is not indicative of the results of operations that would have been achieved if the acquisition had taken place at the beginning of each period presented nor is it indicative of future results. We did not disclose the revenue and income of First Biomedical separately as it is not practical since the operations are already substantially integrated. The following pro forma financial information for all periods presented also includes the pro forma depreciation and amortization charges from acquired tangible and intangible assets, and related tax effects:
| Year Ended December, | ||||||||
| 2010 | 2009 | |||||||
| Net revenues |
$ | 52,316 | $ | 48,741 | ||||
| Net (loss) income |
(1,511 | ) | 1,305 | |||||
| (Loss) earnings per share — basic |
(0.08 | ) | 0.07 | |||||
| (Loss) earnings per share — diluted |
(0.08 | ) | 0.07 | |||||
InfuSystem Acquisition — Additional Contingent Payment
The Stock Purchase Agreement related to the acquisition of InfuSystem provides for a potential additional payment of up to $12.0 million, or the earn-out, to I-Flow in 2011, provided that certain consolidated net revenue growth targets related to the Company’s operations are met. Any amounts ultimately paid out in 2011 per the earn-out would increase Goodwill at the time of payment. No additional payment will be made unless the Company achieves consolidated net revenue compounded annual growth rate (CAGR) of at least 40% over the three-year period. The consolidated net revenue CAGR for the three-year period ended December 31, 2010, as compared to InfuSystem’s 2007 net revenues, was 22% and therefore, there will be no additional payment made related to the acquisition.
| 4. | Property and Equipment |
Property and equipment consisted of the following as of December 31, 2010 and 2009 (amounts in thousands):
| 2010 | 2009 | |||||||
| Pump equipment |
$ | 28,037 | $ | 20,142 | ||||
| Furniture, fixtures, and equipment |
1,894 | 1,832 | ||||||
| Accumulated depreciation |
(13,259 | ) | (8,475 | ) | ||||
| Total |
$ | 16,672 | $ | 13,499 | ||||
46
Included in pump equipment above is $4.6 million and $2.7 million, as of December 31, 2010 and 2009, respectively, worth of pumps obtained under various capital leases. Included in accumulated depreciation above are $723 thousand and $278 thousand, as of December 31, 2010 and 2009, respectively, associated with the same capital leases. Under the terms of all such capital leases, the Company does not presently hold title to these pumps, and will not obtain title until such time as the capital lease obligations are settled in full.
Depreciation expense for 2010, 2009 and 2008 was $5.4 million, $4.1 million and $3.9 million, respectively, which was recorded in cost of revenues and general and administrative expenses, for pump equipment and other fixed assets, respectively.
| 5. | Goodwill and Intangible Assets |
Goodwill
Goodwill arising from business combinations represents the excess of the purchase price over the estimated fair value of the net assets of the businesses acquired. The goodwill amount for the October 25, 2007 acquisition of InfuSystem is $56.6 million, and is based upon the final valuation analysis. The goodwill amount for the June 15, 2010 acquisition of First Biomedical is $7.5 million, and is based upon the final valuation analysis.
Impairment Testing
As of October 31, 2010, the Company performed its annual impairment test pursuant to ASC 350, “Intangibles — Goodwill and Other.” The fair value of the Company’s single reporting unit was estimated using a valuation model that combined an income and market approach, utilizing the discounted cash flow and guideline public company methods, respectively, which indicated that the fair value of its net assets exceeded the carrying value by less than 10%. Based on the results of the valuation, the Company determined there was no impairment of goodwill. No events have occurred subsequent to October 31, 2010 that indicates impairment may have occurred.
The estimated fair value of the Company’s net assets is dependent on several significant assumptions, including management’s projections of future earnings, cost of capital or discount rate and terminal value growth rates. Assumptions related to future cash flows and discount rates involve significant management judgment and are subject to significant uncertainty.
Although the Company’s cash flow forecasts used in the discounted cash flow approach are based on assumptions that are consistent with plans and estimates the Company is using to manage the underlying business, there is significant judgment in projecting the cash flows attributable to the underlying business. If actual revenue growth, profit margins, selling, general and administrative (SGA) expenses, liquidity, capital spending or market conditions should differ significantly from the assumptions included in the Company’s business outlook used in the cash flow models, the fair value of its net assets could fall below the carrying value and impairment charges could be required to write down goodwill to its fair value.
The relationship of the Company’s market capitalization to the carrying value of its net assets can impact estimates of these assumptions, and can therefore impact the Company’s judgment as to the fair value of its reporting unit when performing goodwill impairment tests. During 2010, the Company’s market capitalization remained fairly consistent with such experience in 2009. The Company evaluated the movement in its stock price along with its 2010 performance relative to expectations. In addition, the Company assessed several unique factors; a thinly traded, closely held, illiquid stock, an “overhang” created by having a significant amount of warrants outstanding (see Note 6), and a limited research or analyst coverage. The implied control premium was within the range of market control premiums paid in transactions of companies in the healthcare industry during the past three years. Based on this evaluation, the Company concluded that neither the market capitalization at October 31, 2010, nor the change vs. the prior year, were definitive indicators of impairment.
47
As the Company’s warrants expire in April 2011 and its common stock was listed on the NY AMEX in December 2010, the Company will monitor the impact of these factors as well as control premiums in the healthcare industry, operational performance measures, general economic conditions and its market capitalization. A downward trend in one or more of these factors could cause the Company to reduce the estimated fair value of its reporting unit and recognize a corresponding impairment of goodwill in connection with a future goodwill impairment test.
The Company tests non-amortizable intangible assets (i.e., trade names) for impairment in accordance with ASC 350. The Company performed the annual impairment test at October 31, 2010, and determined there was no impairment. No events have occurred subsequent to October 31, 2010 that indicates impairment may have occurred. The intangible assets resulting from the October 25, 2007 acquisition of InfuSystem are based upon the final valuation analysis. The intangible assets resulting from the June 15, 2010 acquisition of First Biomedical are based upon the final valuation analysis.
Identifiable Intangible Assets
The carrying amount and accumulated amortization of intangible assets as of December 31, 2010 and December 31, 2009 were as follows (in thousands):
| December 31, 2010 |
December 31, 2009 |
|||||||
| Nonamortizable intangible assets |
||||||||
| Trade names |
$ | 5,500 | $ | 5,500 | ||||
| Amortizable intangible assets |
||||||||
| Physician and customer relationships |
32,400 | 27,400 | ||||||
| Non-competition agreements |
760 | — | ||||||
| Software |
980 | — | ||||||
| Total nonamortizable and amortizable intangible assets |
39,640 | 32,900 | ||||||
| Less accumulated amortization |
(6,388 | ) | (3,989 | ) | ||||
| Total identifiable intangible assets |
$ | 33,252 | $ | 28,911 | ||||
Amortization expense for intangible assets for the years ended December 31, 2010 and 2009 was $2.3 million and $1.8 million, respectively, which was recorded in operating expenses. Expected annual amortization expense for intangible assets recorded as of December 31, 2010 is as follows (in thousands):
| 2011 | 2012 | 2013 | 2014 | 2015 | ||||||||||||||||
| Amortization expense |
$ | 2,607 | $ | 2,576 | $ | 2,445 | $ | 2,342 | $ | 2,312 | ||||||||||
| 6. | Warrants and Derivative Financial Instruments |
The Company has determined that the warrants discussed in Note 2, issued in connection with the IPO including the Overallotment Units, should be classified as liabilities in accordance with ASC 815. Therefore, the fair value of each instrument must be recorded as a liability on the Company’s balance sheet. Changes in the fair values of these instruments are reflected as adjustments to the amount of the recorded liabilities, and the corresponding gain or loss is recorded in the Company’s statement of operations within “Gain (loss) on derivatives”. At the date of the conversion of each warrant or portion thereof, or exercise of the warrants or portion thereof, as the case may be, the corresponding liability is reclassified as equity.
On February 16, 2010 the Company announced an Offer to Exchange common stock for outstanding warrants. At the time, the Company had 35,108,219 outstanding warrants. The exchange offer expired on
48
March 17, 2010. Holders of the Company’s warrants had the option to exchange their warrants for either One (1) share of Common Stock for every thirty-five (35) Warrants tendered, or One (1) share of Common Stock for every twenty-five (25) Warrants tendered, provided the recipient agreed to be subject to a lock-up provision precluding transfer of the shares of Common Stock received for six months following the expiration of the Exchange Offer. The lock-up provision expired in September 2010. Based on the final count, 25,635,723 Warrants were properly tendered; 24,766,700 were tendered for shares of Common Stock subject to a lock-up, and 869,023 were tendered for unrestricted shares of Common Stock. Under the terms of the Exchange Offer, the Company issued an aggregate of 1,015,489 shares of Common Stock in exchange for the tendered Warrants. There are 8,329,638 publicly held warrants (issued in connection with the IPO) and 1,142,858 privately held warrants remaining after the exchange.
The fair value of the Company’s 8,329,638 and 33,750,502 warrants issued in connection with the IPO outstanding at December 31, 2010 and December 31, 2009, respectively, were liabilities of $83,000 or $0.01 per warrant and $2,025,000 or $0.06 per warrant, respectively and are included in derivative liabilities within the Company’s balance sheet.
On June 11, 2010, the Company terminated the single interest rate swap agreement that fixed its LIBOR-based variable rate on the Kimberly-Clark (I-Flow) loan. The interest rate swap was terminated through a cash settlement in the amount of $365 thousand, which was the fair value of the interest rate swap as of the date of the termination. The fair value of the Company’s interest rate swap outstanding at December 31, 2009 was a liability of $645 thousand. The Company elected not to designate the swap as a cash flow hedge, in accordance with ASC 815. The fair value of the swap was therefore shown on the Company’s consolidated balance sheet and the unrealized changes in the value of the swap are shown in the Company’s consolidated statement of operations within “Gain (loss) on derivatives”.
On July 20, 2010, the Company entered into a single interest rate swap with a July 30, 2010 effective date. The interest rate swap agreement, which expires in June 2014, had a notional value of $18.3 million on December 31, 2010, which represented approximately 65% of the outstanding underlying debt, and a fixed rate of 1.40%. The fair value of the interest rate swap outstanding at December 31, 2010 was a liability of $100 thousand. The Company has designated the swap as a cash flow hedge. In accordance with ASC 815, the fair value of the swap is shown on the Company’s consolidated balance sheet within derivative liabilities, unrealized changes in the value are included in other comprehensive income within the stockholders’ equity section on the Company’s consolidated balance sheet, and any realized changes are included in the Company’s consolidated statement of operations within interest expense.
The following table presents the fair values of the Company’s derivative instruments as of (in thousands):
| Description |
Balance Sheet Location |
December 31, 2010 |
December 31, 2009 |
|||||||
| Derivative Designated as a Cash Flow |
||||||||||
| Interest rate swap |
Derivative liabilities | $ | 100 | $ | — | |||||
| Derivatives Not Designated as Hedging |
||||||||||
| Warrants |
Derivative liabilities | 83 | 2,025 | |||||||
| Interest rate swap |
Derivative liabilities | — | 645 | |||||||
| Total |
$ | 183 | $ | 2,670 | ||||||
49
The following table presents the pretax impact that changes in the fair values of derivatives designated as hedging instruments had on Accumulated Other Comprehensive Income (AOCI) and earnings during the year ended December 31, 2010 (in thousands):
| Description |
Loss Recognized in OCI |
Location of Gain (Loss) Reclassified from AOCI into Income (Effective Portion) |
Gain (Loss) Reclassified from AOCI into Income (Effective Portion) |
Location of Gain (Loss) Recognized in Income (Ineffective Portion and Amount Excluded from Effectiveness Testing) |
Gain (Loss) Recognized in Income (Ineffective Portion and Amount Excluded from Effectiveness Testing) |
|||||||||||||
| Interest rate swap |
$ | 100 | Gain (loss) on derivatives |
$ | — | |
Gain (loss) on derivatives |
|
$ | — | ||||||||
| Total |
$ | 100 | $ | — | $ | — | ||||||||||||
The following table presents the pretax gains (losses) that changes in the fair values of derivatives not designated as hedging instruments had on earnings during the year ended December 31, 2010 and 2009 (in thousands):
| Description |
Location of Gain (Loss) Recognized |
December 31, 2010 |
December 31, 2009 |
|||||||
| Warrants |
Gain (loss) on derivatives | $ | (73 | ) | $ | (506 | ) | |||
| Interest rate swap |
Gain (loss) on derivatives | 280 | 428 | |||||||
| Total |
$ | 207 | $ | (78 | ) | |||||
The following tables present the methods used to establish fair value measurements for each of the derivatives (in thousands):
| Fair Value Measurements at Reporting Date Using | ||||||||||||||||
| Description |
December 31, 2010 |
Quoted Prices in Active Markets for Identical Liabilities (Level 1) |
Significant Other Observable Inputs (Level 2) |
Significant Unobservable Inputs (Level 3) |
||||||||||||
| Warrant liability |
$ | 83 | $ | 83 | $ | — | $ | — | ||||||||
| Interest rate swap liability |
100 | — | 100 | — | ||||||||||||
| Total |
$ | 183 | $ | 83 | 100 | — | ||||||||||
| Fair Value Measurements at Reporting Date Using | ||||||||||||||||
| Description |
December 31, 2009 |
Quoted Prices in Active Markets for Identical Liabilities (Level 1) |
Significant Other Observable Inputs (Level 2) |
Significant Unobservable Inputs (Level 3) |
||||||||||||
| Warrant liability |
$ | 2,025 | $ | 2,025 | $ | — | $ | — | ||||||||
| Interest rate swap liability |
$ | 645 | — | 645 | — | |||||||||||
| Total |
$ | 2,670 | $ | 2,025 | $ | 645 | — | |||||||||
50
| 7. | Debt and other Long-term Obligations |
On June 15, 2010, the Company entered into a credit facility with Bank of America, N.A. as Administrative Agent, and KeyBank National Association as Documentation Agent. The facility consists of a $30.0 million term loan and a $5.0 million revolving credit facility, both of which mature in June 2014. Interest on the term loan is payable at the Company’s choice of LIBOR plus 4.5%, or the Bank of America prime rate plus 3.5%. As of December 31, 2010, interest was payable at LIBOR plus 4.5%, which equaled approximately 4.76%.
Proceeds from the term loan were used to repay the outstanding balance of the Company’s debt held by Kimberly-Clark (I-Flow), as well as contribute to the acquisition consideration for First Biomedical. As of December 31, 2009, the rate in effect for the Kimberly-Clark (I-Flow) loan was 8.5%.
As of December 31, 2010, the Company had a letter of credit in the amount of $81 thousand outstanding, leaving $4.9 million available on its revolving credit facility.
The term loan is collateralized by substantially all of the Company’s assets and requires the Company to comply with covenants, including but not limited to, financial covenants relating to satisfaction of a total leverage ratio, a fixed charge coverage ratio, and an annual limit on capital expenditures, including capital leases. As of December 31, 2010, the Company believes it was in compliance with all such covenants.
In conjunction with the new credit facility, the Company incurred deferred debt issuance costs of $808 thousand. These costs are recognized in income using the effective interest method through the maturity date of June 15, 2014. Amortization of these costs for the year ended December 31, 2010 was $149 thousand, which was recorded in interest expense. Also, the Company incurred deferred debt issuance costs in 2007 in conjunction with the Kimberly-Clark (I-Flow) loan. The remaining unamortized I-Flow debt costs were completely amortized when the term loan was paid in full on June 15, 2010. Total deferred debt amortization expense for the year ended December 31, 2010 was $980 thousand.
In conjunction with the acquisition of First Biomedical, the Company entered into a subordinated promissory note with the former majority shareholder of First Biomedical (the Seller) in the amount of $750 thousand. In accordance with the note, the Company will pay the Seller in equal installments over 24 months, which includes annual interest of 5%. As of December 31, 2010 the outstanding principal due on the note was $569 thousand.
The Company sometimes enters into capital leases to finance the purchase of ambulatory infusion pumps. The pumps are capitalized into property and equipment at their fair market value, which equals the value of the future minimum lease payments, and are depreciated over the useful life of the pumps.
Maturities on the loans and capital lease are as follows (in thousands):
| 2011 | 2012 | 2013 | 2014 | Total | ||||||||||||||||
| Term Loan |
$ | 4,125 | $ | 4,500 | $ | 4,875 | $ | 14,625 | $ | 28,125 | ||||||||||
| Seller Note |
375 | 194 | — | — | 569 | |||||||||||||||
| Capital Lease |
1,051 | 1,158 | 1,078 | 216 | 3,503 | |||||||||||||||
| Total |
$ | 5,551 | $ | 5,852 | $ | 5,953 | $ | 14,841 | $ | 32,197 | ||||||||||
51
| 8. | Income Taxes |
The components of consolidated provision for income taxes for the years ended December 31, 2010, 2009, and 2008 are as follows:
| 2010 | 2009 | 2008 | ||||||||||
| Provision for Federal income taxes — |
||||||||||||
| Current |
$ | (248 | ) | $ | (1,306 | ) | $ | (165 | ) | |||
| Deferred |
(1,618 | ) | 2,205 | 877 | ||||||||
| Total provision for Federal income taxes |
(1,866 | ) | 899 | 712 | ||||||||
| Provision for state and local income taxes — |
||||||||||||
| Current |
92 | 29 | 137 | |||||||||
| Deferred |
346 | 49 | 58 | |||||||||
| Total provision for state and local income taxes |
438 | 78 | 195 | |||||||||
| Provision for foreign income taxes — |
||||||||||||
| Current |
57 | — | — | |||||||||
| Deferred |
— | — | — | |||||||||
| Total provision for foreign income taxes |
57 | — | — | |||||||||
| Consolidated (benefit) expense for income taxes |
$ | (1,371 | ) | $ | 977 | $ | 907 | |||||
The significant components of net deferred income taxes as of December 31, 2010 and 2009 are as follows:
| 2010 | 2009 | |||||||
| Deferred Federal income tax assets — |
||||||||
| Bad debt reserves |
$ | 98 | $ | 99 | ||||
| Stock based compensation |
1,078 | 249 | ||||||
| Interest Rate Swap |
— | 219 | ||||||
| Net Operating Loss |
2,762 | 1,780 | ||||||
| Accrued Compensation |
273 | 178 | ||||||
| Alternative Minimum Tax Credit |
42 | 42 | ||||||
| Inventory |
80 | 6 | ||||||
| Other |
5 | 2 | ||||||
| Valuation Allowance |
— | (867 | ) | |||||
| Total deferred Federal income tax assets |
4,338 | 1,708 | ||||||
| Deferred Federal income tax liabilities — |
||||||||
| Depreciation and asset basis differences |
(1,998 | ) | (1,653 | ) | ||||
| Amortization |
(6,367 | ) | (3,070 | ) | ||||
| Other |
(33 | ) | (67 | ) | ||||
| Total deferred Federal income tax liabilities |
(8,398 | ) | (4,790 | ) | ||||
| Net deferred Federal income tax liability |
(4,060 | ) | (3,082 | ) | ||||
| Net deferred state and local income tax liability |
(581 | ) | (107 | ) | ||||
| Net deferred income taxes |
$ | (4,641 | ) | $ | (3,189 | ) | ||
52
The classification of net deferred income taxes as of December 31, 2010 is summarized as follows:
| Current | Long-term | Total | ||||||||||
| Deferred tax assets |
$ | 1,185 | $ | 3,661 | $ | 4,846 | ||||||
| Deferred tax liabilities |
(38 | ) | (9,449 | ) | (9,487 | ) | ||||||
| Net deferred income taxes |
$ | 1,147 | $ | (5,788 | ) | $ | (4,641 | ) | ||||
The classification of net deferred income taxes as of December 31, 2009 is summarized as follows:
| Current | Long-term | Total | ||||||||||
| Deferred tax assets |
$ | 194 | $ | 1,573 | $ | 1,767 | ||||||
| Deferred tax liabilities |
(69 | ) | (4,887 | ) | (4,956 | ) | ||||||
| Net deferred income taxes |
$ | 125 | $ | (3,314 | ) | $ | (3,189 | ) | ||||
The reconciliations of the effective income tax rate to the federal statutory rate are as follows:
| 2010 | 2009 | 2008 | ||||||||||
| Federal income tax provision at the statutory rate |
34.00 | % | 34.00 | % | 34.00 | % | ||||||
| State and local income taxes, net of related Federal taxes |
1.25 | % | 4.60 | % | 1.78 | % | ||||||
| Foreign income taxes, net of related Federal taxes |
(1.96 | )% | — | — | ||||||||
| Effect of change in state tax rate |
(13.21 | )% | — | 0.02 | % | |||||||
| Other Permanent Differences |
(2.71 | )% | 2.49 | % | 0.47 | % | ||||||
| Non-deductible loss (gain) on warrant liability |
(0.98 | )% | 9.76 | % | (33.27 | )% | ||||||
| Non-deductible transaction costs |
(9.46 | )% | — | — | ||||||||
| Valuation allowance |
32.42 | % | 6.98 | % | (32.56 | )% | ||||||
| Stock Based Compensation |
6.29 | % | — | 23.53 | % | |||||||
| Prior year adjustments |
1.57 | % | (2.40 | )% | 14.37 | % | ||||||
| Effective income tax rate |
47.21 | % | 55.43 | % | 8.34 | % | ||||||
The Company’s realization of its deferred tax assets is dependent upon many factors, including, but not limited to, the Company’s ability to generate sufficient taxable income. Certain deferred tax liabilities can also be considered as a source of future taxable income including those resulting from the acquisition. In prior years the Company had deferred tax assets to which a full valuation allowance was applied. Based upon the weight of available evidence, it was more likely than not that some portion or all of the deferred tax assets would not be realized. During the year ended December 31, 2010, as a result of a review of the Company’s earnings history, existing deferred tax liabilities including those resulting from the First Biomedical acquisition, the Company has removed the valuation allowance previously applied against the net deferred tax asset.
Following is an analysis of the deferred tax asset valuation allowance for InfuSystem Holdings, Inc. for the years ended December 31, 2010, 2009, and 2008 ($000’s):
| Balance at beginning of Period |
Charged to costs and expenses |
Deductions | Balance at end of Period |
|||||||||||||
| Valuation Allowance — 2010 |
$ | 940 | $ | (940 | ) | $ | — | $ | — | |||||||
| Valuation Allowance — 2009 |
$ | 785 | $ | (458 | ) | $ | 613 | $ | 940 | * | ||||||
| Valuation Allowance — 2008 |
$ | 4,401 | $ | 5 | $ | (3,621 | ) | $ | 785 | |||||||
| * | Includes $867,000 and $73,000 in valuation allowance for federal and state income taxes, respectively. |
53
On June 15, 2010, the Company acquired the stock of First Biomedical, Inc. In accordance with ASC 805, “Business Combinations”, the fair value of the consideration was allocated to the net assets acquired adjusting the book value of the acquired assets to their fair market value. As this was a stock acquisition, the Company’s tax basis of the assets acquired did not change. This differential resulted in the recording of deferred tax liabilities of $2,754,000 in connection with the First Biomedical assets. These new deferred tax liabilities, which are a source of future taxable income, provide strong evidence in support of the valuation allowance reversal as described above.
| 9. | Related Party Transactions |
In 2006, the Company reserved in its treasury 2,000,000 shares of common stock for issuance to Sean McDevitt and 416,666 shares of common stock for issuance to Pat LaVecchia. The consummation of the acquisition of InfuSystem, Inc. resulted in 925,531 of these shares being issued at October 25, 2007. Of the remaining 1,491,135 shares, 257,091 were issued in 2008 and 1,234,044 were issued in February 2009.
During the year ended December 31, 2010, the Company granted 3,225,000 shares to members of the Board of Directors and Officers, and 1,304,250 shares vested and were issued to members of the Board of Directors and Officers. During the year ended December 31, 2009, there were no shares granted to members of the Board of Director or Officers, and 157,709 shares vested and were issued to members of the Board of Directors and Officers. During the year ended December 31, 2008 the Company granted 471,000 shares to members of the Board of Directors and Officers, and 137,500 shares vested and were issued to members of the Board of Directors and Officers. The Company recognized $5.4 million, $399 thousand and $791 thousand in stock based compensation related to members of the Board of Directors and Officers during the years ended December 31, 2010, 2009 and 2008, respectively.
Effective September 7, 2009, Steve Watkins resigned as Chief Executive Officer and Director of the Company.
Prior to the Company’s acquisition of InfuSystem, InfuSystem had been providing billing and collection services to I-Flow for its ON-Q® product. On October 25, 2007, InfuSystem and I-Flow entered into an Amended and Restated Services Agreement (the “Services Agreement”) pursuant to which InfuSystem agreed to continue to provide I-Flow with these services, and I-Flow agreed to pay InfuSystem a monthly service fee. The service was discontinued effective August 31, 2009. During the year ended December 31, 2009, the Company recorded revenues $160 thousand from this arrangement. There was no outstanding receivable amount due as of December 31, 2009. There were no related revenues recorded for the year ended December 31, 2010.
As of December 31, 2009, the Company had $4.9 million payable to Kimberly-Clark (I-Flow) within current portion of long-term debt and $16.8 million payable to Kimberly-Clark (I-Flow) within long-term debt.
On October 19, 2010, the Company facilitated the sale, on behalf of Kimberly-Clark, of 2,789,203 InfuSystem common stock shares held by Kimberly-Clark (I-Flow) through a public secondary offering. This represented 100% of the InfuSystem shares held by Kimberly-Clark (I-Flow). As of October 19, 2010, Kimberly-Clark (I-Flow) is no longer considered a related party. Transaction costs associated with this secondary offering were paid for by Kimberly-Clark (I-Flow).
In connection with the warrant exchange as described in Note 6 to these consolidated financial statements, three present Company board members exchanged 186,287 privately held warrants under the lock-up provision for 7,451 shares of common stock.
As described in Note 7 to these consolidated financial statements, in accordance with the terms of the Stock Purchase Agreement with First Biomedical, the Company entered into a subordinated promissory note with the former majority shareholder of First Biomedical (the Seller) in the amount of $750 thousand. In accordance with
54
the note, the Company will pay the Seller in equal installments over 24 months, which includes annual interest of 5%. As of December 31, 2010 the outstanding principal due on the note was $569 thousand. The Seller is a current employee of the Company, and is subject to an employment agreement. Also, the Seller owns Jan-Mar LLC and is the principal owner of the CW Investment Group LLC. In accordance with the Stock Purchase Agreement, the Company entered into operating lease agreements with Jan-Mar LLC and the CW Investment Group LLC, each of which owns one of the two office buildings utilized by First Biomedical in Olathe, Kansas. The terms of each lease is thirty six months, commencing on July 1, 2010. Rent will be paid monthly in the amount of $5 thousand to Jan-Mar LLC and $3 thousand to the CW Investment Group LLC.
| 10. | Commitments and Contingencies |
Certain of the Company’s directors committed to purchase up to $1.0 million of the Company’s warrants from the Company in a private placement at a price of $.70 per warrant subsequent to the filing of the preliminary proxy statement seeking stockholder approval of the acquisition of InfuSystem. Such officers and directors agreed not to sell or transfer the warrants until after the Company consummated a business combination. The warrants have an exercise price of $5.00 per share of common stock and became exercisable commencing on October 25, 2007, the acquisition date, and expire April 11, 2011 or earlier upon redemption by the Company. The Company may call the warrants for redemption in whole and not in part at a price of $0.01 per warrant at anytime after the warrant becomes exercisable. The warrants cannot be redeemed unless the holder receives written notice not less than 30 days prior to the redemption and if and only if, the reported last price of the common stock equals or exceeds $8.50 per share for any 20 trading days within a 30 day period ending on the third day of business prior to the notice of redemption to warrant holders. The Company has fully reserved the shares underlying the warrants as authorized but not issued. The warrants issued and sold in 2006 and 2007 were not registered under the Securities Act of 1933, as amended (the Securities Act). As a result, the warrants and the common stock issuable upon exercise of the warrants may not be sold unless they have been registered pursuant to a registration statement filed under the Securities Act or pursuant to an available exemption from the registration requirements of the Securities Act as evidenced by an opinion of counsel reasonably satisfactory to the Company. There are 1,142,858 privately held warrants remaining after the exchange as discussed in Note 6 to these consolidated financial statements.
The Company is involved in legal proceedings arising out of the ordinary course and conduct of our business, the outcomes of which are not determinable at this time. We have insurance policies covering such potential losses where such coverage is cost effective. In the Company’s opinion, any liability that might be incurred by us upon the resolution of these claims and lawsuits will not, in the aggregate, have a material adverse effect on the Company’s consolidated financial position, results of operations or cash flows
Effective September 7, 2009, Steve Watkins resigned as Chief Executive Officer and Director of the Company. In connection with his resignation, the Company entered into a separation agreement with Mr. Watkins in which the Company will pay Mr. Watkins his annual base salary of $311 thousand for a period of two years following the resignation date in accordance with the Company’s regular payroll practices. Also, the Company agreed to pay a bonus in the amount of $150 thousand for the 2009 calendar year within thirty days of the resignation date; such amount was paid in October 2009. The Company will continue to pay for Mr. Watkins’ existing health insurance benefits for a period of two years following the resignation date. Additionally, any unvested portions of Mr. Watkins’ stock options and restricted share grants vested pro rata based upon his services to the Company as Chief Executive Officer during the 2009 calendar year.
As of December 31, 2010, the Company had approximate minimum future operating lease commitments of (in thousands):
| 2011 |
2012 | 2013 | 2014 | 2015 | ||||||||||||
| $481 | $ | 360 | $ | 236 | $ | 195 | $ | 200 | ||||||||
55
| 11. | Share-based Compensation |
2007 Stock Incentive Plan
In 2007, the Company adopted the 2007 Stock Incentive Plan providing for the issuance of a maximum of 2,000,000 shares of common stock in connection with the grant of stock-based or stock-denominated awards. During 2010 and 2009, the Company granted restricted shares and stock options. As of December 31, 2010, 68,000 common shares remained available for future grant under the 2007 Stock Incentive Plan.
During the year ended December 31, 2010 the Company granted restricted shares both under the Plan and outside of it, and during the year ended December 31, 2009 the Company granted restricted shares and stock options under the Plan.
During the year ended December 31, 2010, the Company granted 3,440,000 restricted shares. Of the total shares granted, 1,440,000 entitle a holder to receive, at the end of a vesting period, a specified number of shares of the Company’s common stock. The remaining 2,000,000 shares granted entitle the holder to receive common stock when the shares vest based upon certain market conditions tied to the Company’s stock price, or certain performance conditions including a change in control.
Restricted Shares
Restricted shares entitle the holder to receive, upon meeting certain vesting criteria, a specified number of shares of the Company’s common stock. Stock-based compensation cost of restricted shares is measured by the market value of the Company’s common stock on the date of grant. Compensation cost associated with certain restricted share grants also takes into account market conditions in its measurement. The following table summarizes restricted share activity for the years ended December 31, 2010 and 2009:
| Number of shares (In thousands) |
Weighted average grant date fair value |
|||||||
| Unvested at January 1, 2009 |
521 | $ | 2.94 | |||||
| Granted |
133 | $ | 2.66 | |||||
| Vested |
(213 | ) | $ | 2.93 | ||||
| Vested shares foregone to satisfy minimum statutory withholding |
(52 | ) | $ | 2.86 | ||||
| Forfeitures |
(65 | ) | $ | 2.87 | ||||
| Unvested at December 31, 2009 |
324 | $ | 2.86 | |||||
| Granted |
3,440 | $ | 2.50 | |||||
| Vested |
(1,408 | ) | $ | 2.56 | ||||
| Vested shares forgone to satisfy minimum statutory withholding |
(67 | ) | $ | 2.78 | ||||
| Forfeitures |
(115 | ) | $ | 2.66 | ||||
| Unvested at December 31, 2010 |
2,174 | $ | 2.51 | |||||
As of December 31, 2010, there was $7.5 million of pre-tax total unrecognized compensation cost related to non-vested restricted shares, which will be adjusted for future forfeitures. The Company expects to recognize such cost over a period of approximately 14 years.
Stock Options
There were no stock options granted during the year ended December 31, 2010. During the year ended December 31, 2009, the Company granted 30 thousand stock options at an exercise price of $1.85 per share which was the market price on the date of grant.
56
Share-based compensation expense was determined based on the fair value of the options. The fair value of the options was calculated using the Black Scholes pricing model based on the following assumptions:
| 2009 | ||
| Expected life |
2.5 years | |
| Risk free interest rate |
1.38% - 1.42% | |
| Volatility |
35% - 37% | |
| Dividend yield |
0% |
The following table summarizes stock option activity for the year ended December 31, 2009:
| Number of options (In thousands) |
Weighted average exercise price |
|||||||
| Unvested at January 1, 2009 |
300 | $ | 2.90 | |||||
| Granted |
30 | $ | 1.85 | |||||
| Vested |
(130 | ) | $ | 2.66 | ||||
| Forfeitures |
(200 | ) | $ | 2.90 | ||||
| Unvested at December 31, 2009 |
— | $ | N/A | |||||
Stock-based compensation expense
The following table shows the total stock-based compensation expense, which is included in selling, general and administrative expenses, related to all of the Company’s equity awards in accordance with ASC 718 (in thousands):
| December 31, | ||||||||||||
| 2010 | 2009 | 2008 | ||||||||||
| Restricted share expense |
$ | 5,853 | * | $ | 700 | $ | 1,415 | |||||
| Stock option expense |
— | 53 | 135 | |||||||||
| Total stock-based compensation expense |
$ | 5,853 | $ | 753 | $ | 1,550 | ||||||
| * | Includes $2.1 million expense for a tax gross-up liability associated with certain restricted share grants. |
Common Share Repurchase Program
In November of 2010, our board of directors authorized a share repurchase program of up to $2 million of our outstanding common shares. The repurchase program will be funded by our available cash balance.
Stock repurchases may be made through open market transactions, negotiated purchases or otherwise, at times and in such amounts as our management deems to be appropriate. The timing and actual number of shares repurchased will depend on a variety of factors, including price, financing and regulatory requirements, as well as other market conditions. The program does not require us to repurchase any specific number of shares or to complete the program within a specific period of time.
During the year ended December 31, 2010, we repurchased 46,000 shares at an average price of $2.46 per share at a cost of $114,000
| 12. | Employee Benefit Plans |
The Company has defined contribution plans in which the company contributes a certain percentage of employee contributions. Such Company matching contributions totaled $196 thousand, $67 thousand and $59 thousand for the years ended December 31, 2010, 2009 and 2008, respectively. The Company does not provide other post-retirement or post-employment benefits to its employees.
57
| 13. | Unaudited Quarterly Information |
| Quarter Ended | ||||||||||||||||
| (in thousands, except per share data) |
March 31, 2010 |
June 30, 2010 |
September 30, 2010 |
December 31, 2010 |
||||||||||||
| Net revenues |
$ | 10,934 | $ | 10,487 | $ | 12,733 | $ | 13,075 | ||||||||
| Gross profit |
8,120 | 7,520 | 8,897 | 9,009 | ||||||||||||
| Sales, general and administrative expenses |
6,628 | 7,774 | 8,069 | 12,013 | ||||||||||||
| Total other (expense) income |
(1,194 | ) | (319 | ) | (359 | ) | (412 | ) | ||||||||
| Income (loss) before income taxes |
298 | (573 | ) | 469 | (3,416 | ) | ||||||||||
| Net (loss) income |
(12 | ) | 144 | 174 | (2,158 | ) | ||||||||||
| (Loss) earnings per share — basic |
0.00 | 0.01 | 0.01 | (0.11 | ) | |||||||||||
| (Loss) earnings per share — diluted |
0.00 | 0.01 | 0.01 | (0.11 | ) | |||||||||||
| Quarter Ended | ||||||||||||||||
| (in thousands, except per share data) |
March 31, 2009 |
June 30, 2009 |
September 30, 2009 |
December 31, 2009 |
||||||||||||
| Net revenues |
$ | 9,227 | $ | 9,173 | $ | 9,902 | $ | 10,662 | ||||||||
| Gross profit |
7,117 | 6,895 | 7,116 | 7,788 | ||||||||||||
| Sales, general and administrative expenses |
5,868 | 5,551 | 5,753 | 6,137 | ||||||||||||
| Total other income (expense) |
(3,628 | ) | 1,155 | (1,395 | ) | 291 | ||||||||||
| Income (loss) before income taxes |
(2,367 | ) | 2,499 | (32 | ) | 1,651 | ||||||||||
| Net income (loss) |
(2,507 | ) | 2,760 | (445 | ) | 966 | ||||||||||
| Earnings (loss) per share — basic |
(0.14 | ) | 0.15 | (0.02 | ) | 0.05 | ||||||||||
| Earnings (loss) per share — diluted |
(0.14 | ) | 0.15 | (0.02 | ) | 0.05 | ||||||||||
58
| Item 9. | Changes in and Disagreements with Accountants on Accounting and Financial Disclosures. |
None.
| Item 9A. | Controls and Procedures. |
Disclosure Controls and Procedures
We maintain disclosure controls and procedures, (as defined in Rules 13a-15(e) and 15d-15(e) under the Securities Exchange Act of 1934, as amended (the Exchange Act) that are designed to ensure that information required to be disclosed in our reports that we file or submit under the Exchange Act is recorded, processed, summarized and reported within the time periods specified in the SEC’s rules and forms and that such information is accumulated and communicated to our management, including our Chief Executive Officer (principal executive officer) and Chief Financial Officer (principal accounting and financial officer), as appropriate, to allow timely decisions regarding required financial disclosure. In designing and evaluating the disclosure controls and procedures, management recognizes that a control system, no matter how well designed and operated, can provide only reasonable, not absolute, assurance that the objectives of the control system are met. Because of the inherent limitations in all control systems, no evaluation of controls can provide absolute assurance that all control issues and instances of fraud, if any, with a company have been detected.
As of the end of the period covered by this Annual Report on Form 10-K, we carried out an evaluation, under the supervision and with the participation of our management, including our Chief Executive Officer and Chief Financial Officer, of the effectiveness of the design and operation of our disclosure controls and procedures (as defined in Rules 13a-15(e) and 15d-15(e) under the Exchange Act) as of December 31, 2010. Based upon that evaluation, our Chief Executive Officer and Chief Financial Officer each concluded that our disclosure controls and procedures are effective, at the reasonable assurance level, as of the end of the period covered by this Annual Report on Form 10-K, as of December 31, 2010.
Management’s Report on Internal Control Over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting as defined in Rule 13a-15(f) of the Exchange Act. Our internal control over financial reporting system is designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with accounting principles generally accepted in the United States of America. All internal control systems, no matter how well designed, have inherent limitations. Therefore, even those systems determined to be effective can provide only reasonable assurance that material misstatements will be prevented or detected on a timely basis. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
Our management assessed the effectiveness of our internal control over financial reporting as of December 31, 2010. The assessment of the related controls at First Biomedical, which was acquired during the fiscal year, was excluded from the assessment. In making this assessment, management used the criteria set forth by the Committee of Sponsoring Organizations of the Treadway Commission (COSO) in Internal Control-Integrated Framework. Based on its assessment, management has concluded that, as of December 31, 2010, our internal control over financial reporting is effective based on those criteria.
This annual report does not include an attestation report of the Company’s independent registered public accounting firm regarding internal control over financial reporting because that requirement under Section 404 of the Sarbanes-Oxley Act of 2002 was permanently removed for non-accelerated filers pursuant to the provisions of Section 989G(a) set forth in the Dodd-Frank Wall Street Reform and Consumer Protection Act enacted into federal law in July 2010.
59
Changes in Internal Control Over Financial Reporting
During the year ended December 31, 2010 the Company began implementing a new Enterprise Resource Planning (ERP) system and certain phases have been completed and modules implemented, including payroll and employee benefits modules. Further implementation and improvements will continue in next year ending December 31, 2010.
There were no other changes to our internal control over financial reporting that occurred during the quarter ended December 31, 2010 that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
| Item 9B. | Other Information. |
None.
| Item 10. | Directors, Executive Officers and Corporate Governance |
The information required by Part III, Item 10 is incorporated herein by reference to our definitive proxy statement relating to the 2011 Annual Meeting of Stockholders to be filed with the SEC within 120 days after the end of the fiscal year covered by this Annual Report on Form 10-K.
| Item 11. | Executive Compensation |
The information required by Part III, Item 11 is incorporated herein by reference to our definitive proxy statement relating to the 2011 Annual Meeting of Stockholders to be filed with the SEC within 120 days after the end of the fiscal year covered by this Annual Report on Form 10-K.
| Item 12. | Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters |
The information required by Part III, Item 12 is incorporated herein by reference to our definitive proxy statement relating to the 2010 Annual Meeting of Stockholders to be filed with the SEC within 120 days after the end of the fiscal year covered by this Annual Report on Form 10-K.
| Item 13. | Certain Relationships and Related Transactions, and Director Independence |
The information required by Part III, Item 13 is incorporated herein by reference to our definitive proxy statement relating to the 2011 Annual Meeting of Stockholders to be filed with the SEC within 120 days after the end of the fiscal year covered by this Annual Report on Form 10-K.
| Item 14. | Principal Accounting Fees and Services |
The information required by Part III, Item 14 is incorporated herein by reference to our definitive proxy statement relating to the 2011 Annual Meeting of Stockholders to be filed with the SEC within 120 days after the end of the fiscal year covered by this Annual Report on Form 10-K.
60
| Item 15. | Exhibits and Financial Statement Schedules |
| (a) | 1. | Financial Statements | ||
| Reference is made to the Index to Financial Statements under Item 8, Part II hereof. | ||||
| 2. | Financial Statement Schedules | |||
| The Financial Statement Schedules have been omitted either because they are not required or because the information has been included in the financial statements or the notes thereto included in this Annual Report on Form 10-K. | ||||
| 3. | Exhibits | |||
| (b) | See Item 15(a)(3) | |||
| (c) | See Item 15(a)(3) | |||
61
Exhibit Index
| Exhibit |
Description of Document | |
| 3.1 | Amended and Restated Certificate of Incorporation (1) | |
| 3.2 | Certificate of Amendment of Amended and Restated Certificate of Incorporation (5) | |
| 3.3 | By-Laws (1) | |
| 3.4 | Amended and Restated By-Laws (2) | |
| 3.5 | Certificate of Amendment of Amended and Restated Certificate of Incorporation (16) | |
| 3.6 | Amended and Restated By-Laws (20) | |
| 4.1 | Specimen Unit Certificate (4) | |
| 4.2 | Specimen Common Stock Certificate (4) | |
| 4.3 | Specimen Warrant Certificate (4) | |
| 4.4 | Form of Warrant Agreement between Registrant and Mellon Investor Services LLC (2) | |
| 4.5 | Form of Purchase Option granted to FTN Midwest Securities Corp. (4) | |
| 4.6 | Form of Warrant issued to Sean McDevitt (7) | |
| 4.7 | Unit Purchase Option Clarification Agreement, dated as of February 9, 2007, by and between Registrant and FTN Midwest Securities Corp. (8) | |
| 4.8 | Warrant, dated as of April 12, 2007, issued to Sean McDevitt (9) | |
| 4.9 | Form of Warrant between Registrant and each of Sean McDevitt, John Voris, Wayne Yetter, Jean Pierre Millon and Erin Enright. (12) | |
| 10.1 | Amended and Restated Registration Rights Agreement, dated as of October 17, 2007 by and among Registrant, Wayne Yetter, John Voris, Jean-Pierre Millon, Erin Enright, Sean McDevitt, Pat LaVecchia and Great Point Partners LLC (22) | |
| 10.2 | Form of Stock Transfer Agency Agreement (2) | |
| 10.3 | Stock Purchase Agreement, dated as of September 29, 2006, by and among Registrant, Iceland Acquisition subsidiaries, Inc., InfuSystem, Inc. and I-Flow Corporation (6) | |
| 10.4 | Amendment No. 1 to Stock Purchase Agreement, dated as of April 30, 2007, by and among Registrant, Iceland Acquisition subsidiaries, Inc., InfuSystem, Inc. and I-Flow Corporation (9) | |
| 10.5 | Amendment No. 2 to Stock Purchase Agreement, dated as of June 29, 2007, by and among Registrant, Iceland Acquisition subsidiaries, Inc., InfuSystem, Inc. and I-Flow Corporation (10) | |
| 10.6 | Amendment No. 3 to Stock Purchase Agreement, dated as of July 31, 2007, by and among Registrant, Iceland Acquisition subsidiaries, Inc., InfuSystem, Inc. and I-Flow Corporation (11) | |
| 10.7 | Amendment No. 4 to Stock Purchase Agreement, dated as of September 18, 2007, by and among Registrant, Iceland Acquisition subsidiaries, Inc., InfuSystem, Inc. and I-Flow Corporation (14) | |
| 10.8 | Subscription Agreement, dated as of April 12, 2007, between Registrant and Sean McDevitt (9) | |
| 10.9 | Form of Subscription Agreement between Registrant and each of Sean McDevitt, John Voris, Wayne Yetter, Jean Pierre Millon and Erin Enright (12) | |
| 10.10 | Memorandum of Intent, dated as of September 12, 2007, by and among Registrant, I-Flow Corporation, InfuSystem, Inc. and Iceland Acquisition subsidiaries, Inc. (13) | |
62
| Exhibit |
Description of Document | |
| 10.11 | Further Agreement Regarding Project Iceland, dated as of October 17, 2007, by and among Registrant, I-Flow Corporation, InfuSystem, Inc. and Iceland Acquisition subsidiaries, Inc. (15) | |
| 10.12 | Acknowledgment and Agreement, dated as of October 8, 2007, by and among Registrant, I-Flow Corporation, InfuSystem, Inc. and Iceland Acquisition subsidiaries, Inc. (16) | |
| 10.13 | Credit and Guaranty Agreement, dated as of October 25, 2007, by and among Registrant, Iceland Acquisition subsidiaries, Inc. and I-Flow Corporation (16) | |
| 10.15 | Employment Agreement, dated as of November 12, 2007, by and between Registrant and Sean Whelan (17) | |
| 10.16 | Employment Agreement, dated as of November 12, 2007, by and between Registrant and Janet Skonieczny (17) | |
| 10.17 | InfuSystem Holdings, Inc. 2007 Stock Incentive Plan (19) | |
| 10.18 | Separation Agreement, dated August 28, 2009, by and between Registrant and Steve Watkins (22) | |
| 10.19 | Share Award Agreement between the InfuSystem Holdings, Inc. and Sean McDevitt (24) | |
| 10.20 | Restricted Stock Award Agreement between Sean Whelan and InfuSystem Holdings, Inc. (25) | |
| 10.21 | Restricted Stock Award Agreement between Jan Skonieczny and InfuSystem Holdings, Inc. (25) | |
| 10.22 | Restricted Stock Award Agreement between Bryan Russo and InfuSystem Holdings, Inc. (25) | |
| 10.23 | Restricted Stock Award Agreement between David Haar and InfuSystem Holdings, Inc. (25) | |
| 10.24 | Restricted Stock Award Agreement between Scott Chesky and InfuSystem Holdings, Inc. (25) | |
| 10.25 | Restricted Stock Award Agreement between Timothy Kopra and InfuSystem Holdings, Inc. (25) | |
| 10.26 | InfuSystem Holdings, Inc. 2007 Stock Incentive Plan (23) | |
| 14.1 | Code of Ethics (3) | |
| 21.1 | Subsidiaries of Registrant (18) | |
| 23.1 | Consent of Deloitte & Touche LLP * | |
| 31.1 | Certification of Chief Executive Officer pursuant to Rule 13a-14 of the Securities Exchange Act of 1934, as amended * | |
| 31.2 | Certification of Principal Accounting Officer pursuant to Rule 13a-14 of the Securities Exchange Act of 1934, as amended * | |
| 32.1 | Certification of Chief Executive Officer pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 * | |
| 32.2 | Certification of Principal Accounting Officer pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 * | |
| * | Filed herewith |
| (1) | Incorporated by reference to Registrant’s Registration Statement on Form S-1 (File No. 333-129035) filed on October 14, 2005. |
| (2) | Incorporated by reference to Amendment No. 1 to Registrant’s Registration Statement on Form S-1 (File No. 333-129035) filed on December 8, 2005. |
| (3) | Incorporated by reference to Amendment No. 2 to Registrant’s Registration Statement on Form S-1 (File No. 333-129035) filed on January 17, 2006. |
| (4) | Incorporated by reference to Amendment No. 3 to Registrant’s Registration Statement on Form S-1 (File No. 333-129035) filed on March 3, 2006. |
| (5) | Incorporated by reference to Registrant’s Current Report on Form 8-K filed on April 24, 2006. |
63
| (6) | Incorporated by reference to Registrant’s Current Report on Form 8-K filed on October 4, 2006. |
| (7) | Incorporated by reference to Registrant’s Current Report on Form 8-K filed on January 3, 2007. |
| (8) | Incorporated by reference to Registrant’s Current Report on Form 8-K filed on February 14, 2007. |
| (9) | Incorporated by reference to Registrant’s Current Report on Form 8-K filed on May 4, 2007. |
| (10) | Incorporated by reference to Registrant’s Current Report on Form 8-K filed on July 5, 2007. |
| (11) | Incorporated by reference to Registrant’s Current Report on Form 8-K filed on August 1, 2007. |
| (12) | Incorporated by reference to Registrant’s Current Report on Form 8-K filed on September 12, 2007. |
| (13) | Incorporated by reference to Registrant’s Current Report on Form 8-K filed on September 13, 2007. |
| (14) | Incorporated by reference to Registrant’s Current Report on Form 8-K filed on September 21, 2007. |
| (15) | Incorporated by reference to Registrant’s Current Report on Form 8-K filed on October 22, 2007. |
| (16) | Incorporated by reference to Registrant’s Current Report on Form 8-K filed on October 31, 2007. |
| (17) | Incorporated by reference to Registrant’s Current Report on Form 8-K filed on November 16, 2007. |
| (18) | Incorporated by reference to Registrant’s Annual Report on Form 10-K filed on March 24, 2008. |
| (19) | Incorporated by reference to Registrant’s Registration Statement on Form S-8 (File No. 333-150066) filed on April 3, 2008. |
| (20) | Incorporated by reference to Registrant’s Current Report on Form 8-K filed on January 22, 2008. |
| (21) | Incorporated by reference to Registrant’s Current Report on Form 8-K filled on September 1, 2009. |
| (22) | Incorporated by reference to Registrant’s Annual Report on Form 10-K filed on March 3, 2009. |
| (23) | Incorporated by reference to Registrant’s Registration Statement on Form S-8 (File No. 333-150066) filed on April 3, 2008. |
| (24) | Incorporated by reference to Registrant’s Current Report on Form 8-K filed April 9, 2010. |
| (25) | Incorporated by reference to Registrant’s Registration Statement on Form S-8 (File No. 333-167914) filed on July 1, 2010. |
64
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) Securities Exchange Act of 1934, the Registrant has duly caused this Report to be signed on its behalf by the undersigned, thereunto duly authorized.
| INFUSYSTEM HOLDINGS, INC. | ||||||||
| Date: March 10, 2011 | By: | /s/ SEAN MCDEVITT | ||||||
| Sean McDevitt | ||||||||
| Chief Executive Officer | ||||||||
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the Registrant and in the capacity and on the dates indicated.
| Date: March 10, 2011 | /s/ SEAN MCDEVITT | |
| Sean McDevitt | ||
| Chief Executive Officer and Director (Principal Executive Officer) | ||
| Date: March 10, 2011 | /s/ JAMES FROISLAND | |
| James Froisland | ||
| Chief Financial Officer (Principal Accounting and Financial Officer) | ||
| Date: March 10, 2011 | /s/ SEAN MCDEVITT | |
| Sean McDevitt | ||
| Chairman of the Board | ||
| Date: March 10, 2011 | /s/ JOHN VORIS | |
| John Voris | ||
| Director | ||
| Date: March 10, 2011 | /s/ PAT LAVECCHIA | |
| Pat LaVecchia | ||
| Director | ||
| Date: March 10, 2011 | /s/ WAYNE YETTER | |
| Wayne Yetter | ||
| Director | ||
| Date: March 10, 2011 | /s/ JEAN-PIERRE MILLON | |
| Jean-Pierre Millon | ||
| Director | ||
| Date: March 10, 2011 | /s/ DAVID DREYER | |
| David Dreyer | ||
| Director | ||
| Date: March 10, 2011 | /s/ JAMES FREDDO | |
| James Freddo | ||
| Director | ||
| Date: March 10, 2011 | /s/ TIM KOPRA | |
| Tim Kopra | ||
| Director | ||
65